EPIDEMIOLOGY
Worldwide, the incidence of HCC in developing nations is over twice the incidence of that in developed countries. In 2000, the age-adjusted incidence of HCC in men was 17.43 per 100,000 population in developing countries compared with only 8.7 per 100,000 population in the United States. Among women, the disparity was also significant (6.77 vs 2.86 per 100,000 population). The highest incidence of HCC is in East Asia, with incidence rates in men of 35 per 100,000 population, followed by Africa and the Pacific Islands.(1)
Mortality figures mirror the incidence figures for HCC. In developing countries, the mortality from HCC in men is more than double that in developed countries (16.86 vs 8.07 per 100,000 population). In Asia and Africa, the mortality figures are 33.5 and 23.73 per 100,000 population, respectively.(1)
Based on results of a reliable hospital-based registry in Pakistan, hepatobiliary cancers are the most common malignancy in adult males and represent 10.7% of all cancers.(2) The age standardized rate for HCC in Pakistan is 7.6 per 100,000 persons per year for males and 2.8 for females.(3-5)
PATHOPHYSIOLOGY(1)
The pathophysiology of HCC has not been definitively elucidated and is clearly a multifactorial event. In 1981, after Beasley linked hepatitis B virus (HBV) infection to HCC development, the cause of HCC was thought to have been identified.(7) However, subsequent studies failed to identify HBV infection as a major independent risk factor, and it became apparent that most cases of HCC developed in patients with underlying cirrhotic liver disease of various etiologies, including patients with negative markers for HBV infection and who were found to have HBV DNA integrated in the hepatocyte genome.
Inflammation, necrosis, fibrosis, and ongoing regeneration characterize the cirrhotic liver and contribute to HCC development. In patients with HBV, in whom HCC can develop in livers that are not frankly cirrhotic, underlying fibrosis is usually present, with the suggestion of regeneration. By contrast, in patients with hepatitis C virus (HCV), HCC invariably presents, more or less, in the setting of cirrhosis. This difference may relate to the fact that HBV is a DNA virus that integrates in the host genome and produces HBV X protein that may play a key regulatory role in HCC development;(8) HCV is an RNA virus that replicates in the cytoplasm and does not integrate in the host DNA.
The disease processes, which result in malignant transformation, include a variety of pathways, many of which may be modified by external and environmental factors and eventually lead to genetic changes that delay apoptosis and increase cellular proliferation (see the image below).
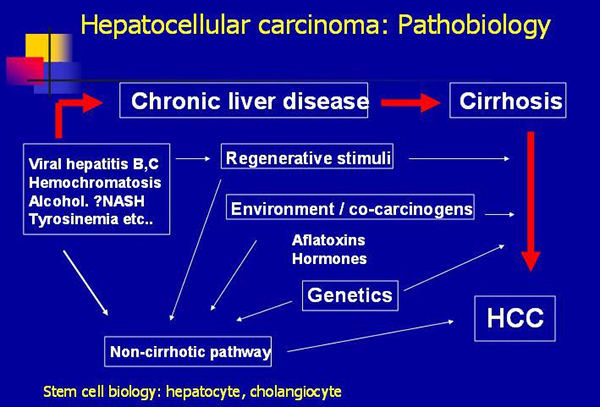
Hepatocellular carcinoma: pathobiology (Courtesy Medscape)
Efforts have been made to elucidate the genetic pathways that are altered during hepatocarcinogenesis.(9) Among the candidate genes involved, the p53, PIKCA, and β-catenin genes appear to be the most frequently mutated in patients with HCC. Additional investigations are needed to identify the signal pathways that are disrupted, leading to uncontrolled division in HCC. Two pathways involved in cellular differentiation (i.e. Wnt-β-catenin, Hedgehog) appear to be frequently altered in HCC. Upregulated WNT signaling appears to be associated with preneoplastic adenomas with a higher rate of malignant transformation.
Additionally, studies of inactivated mutations of the chromatin remodeling gene ARID2 in four major subtypes of HCC are being performed. A total of 18.2% of individuals with HCV-associated HCC in the United States and Europe harbored ARID2 inactivation mutations. These findings suggest that ARID2 is a tumor suppressor gene commonly mutated in this tumor subtype.(10)
Whereas various nodules are frequently found in cirrhotic livers, including dysplastic and regenerative nodules, no clear progression from these lesions to HCC occurs. Prospective studies suggest that the presence of small-cell dysplastic nodules conveyed an increased risk of HCC, but large-cell dysplastic nodules were not associated with an increased risk of HCC. Evidence linking small-cell dysplastic nodules to HCC includes the presence of conserved proliferation markers and the presence of nodule-in-nodule on pathologic evaluation. This term describes the presence of a focus of HCC in a larger nodule of small dysplastic cells.(11)
Some investigators have speculated that HCC develops from hepatic stem cells that proliferate in response to chronic regeneration caused by viral injury.(12) The cells in small dysplastic nodules appear to carry markers consistent with progenitor or stem cells.
NATURAL HISTORY
Natural history of HCC can be split into three distinct phases: (i) ‘molecular’; (ii) ‘preclinical’ and (iii) ‘clinical or symptomatic’ ( Figure 1 ).
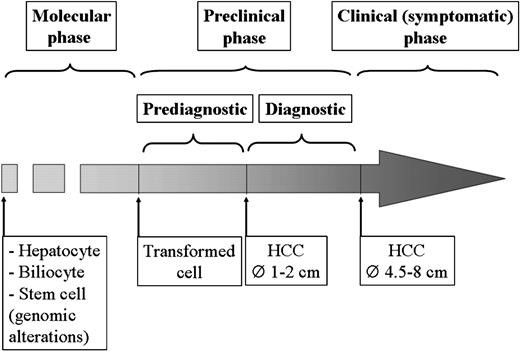
Figure 1: The natural history of HCC can be divided into three distinct phases: (i) molecular, (ii) preclinical and (iii) clinical or symptomatic.
The preclinical phase covers an initial period, in which the tumor is too small to be detected by imaging techniques and a second period (preclinical diagnostic phase), during which the tumor is detectable but still asymptomatic. Finally, the clinical or symptomatic phase starts with the occurrence of symptoms caused by the tumor burdens. In patients with chronic liver disease, HCC usually becomes symptomatic when it reaches 4.5–8 cm.
The molecular phase includes the sequential genomic alterations leading to cell transformation. It has been postulated that the transformed cell can be either a hepatocyte/biliary epithelial or a liver stem cell.(13) The genetic alterations involving differentiated cells (hepatocytes and biliocytes) are thought to confer a growth advantage by promoting proliferation and inhibiting apoptosis, whereas those involving stem cells interfere with the differentiation process. In human carcinogenesis, the time to acquire these genetic changes is unknown.
The preclinical phase covers an initial period, in which the tumor is still too small to be detected by imaging techniques, and a second period (‘preclinical diagnostic’ phase), during which the tumor is detectable but still asymptomatic.
Finally, the clinical or symptomatic phase starts with the occurrence of symptoms caused by the tumor burden: in patients with chronic liver disease, HCC usually becomes symptomatic when it reaches 4.5–8 cm.(14,15)
SIGN AND SYMPTOMS
Patterns of presentation:
There is a range of clinical presentations for patients with HCC, from being asymptomatic to presenting with a life-threatening illness such as variceal hemorrhage. Many patients who develop HCC have no symptoms specifically related to the tumor, especially for those who have been undergoing regular surveillance and have HCC detected at an early stage.
Previously stable patients with cirrhosis may develop features of decompensation (e.g. variceal bleeding or ascites) due to the extension of HCC into the hepatic or portal veins. In other cases, patients without known chronic liver disease or cirrhosis may first develop symptoms (e.g. jaundice, abdominal pain) that are further evaluated with imaging and possibly biopsy that confirms the diagnosis of HCC.
Symptomatic patients:
Patients with advanced lesions may present with mild to moderate upper abdominal pain, weight loss, early satiety, or a palpable mass in the upper abdomen.(16)
Paraneoplastic syndromes: Patients with HCC may occasionally develop a paraneoplastic syndrome that can manifest with the following features which (except for erythrocytosis) are generally associated with a poor prognosis:(17)
- Hypoglycemia: Hypoglycemia, which usually occurs in advanced HCC, is thought to result from the tumor’s high metabolic needs. The hypoglycemia is typically mild and produces no symptoms; however, more severe reductions in the plasma glucose can occur, resulting in lethargy and confusion.
Less than 5 percent of tumors secrete insulin-like growth factor-II, which can cause severe, symptomatic hypoglycemia sometimes early in the course of the disease.(18,19)
- Erythrocytosis: Erythrocytosis in HCC is probably due to tumor secretion of erythropoietin (EPO).(20) Although raised serum EPO levels may be present in up to 23 percent of patients with HCC, elevations in hemoglobin concentration or packed cell volume are uncommon, and most patients are anemic at diagnosis because of other effects of the tumor.(21)
- Hypercalcemia: Hypercalcemia can be present in association with osteolytic metastases, but it may also be seen in the absence of bony metastasis due to secretion of parathyroid hormone-related protein.(22)
- Diarrhea: Patients with HCC may infrequently present with intractable diarrhea and associated electrolyte disturbances (e.g. hyponatremia, hypokalemia, metabolic alkalosis).(23,24) The underlying mechanism is not fully understood, but it may be related to secretion of peptides that cause intestinal secretion. These include vasoactive intestinal polypeptide, gastrin, and peptides with prostaglandin-like immunoreactivity.(25)
- Cutaneous features: Although skin changes are rare in patients with HCC, several cutaneous manifestations have been described; however, none is specific for the diagnosis.(25,26) These include:
- Dermatomyositis may present with a variety of cutaneous findings (e.g. scaly, violaceous papules overlying bony prominences of the hands) and is associated with solid organ malignancies.
- Pemphigus foliaceus is a superficial blistering disease similar to pemphigus vulgaris, except it rarely involves the mucous membranes. Blisters often appear as shallow erosions associated with erythema, scale and crust formation, and the appearance may resemble severe seborrheic dermatitis.
- The sign of Leser-Trélat refers to the sudden appearance of multiple seborrheic keratoses, often with an inflammatory base in association with skin tags and acanthosis nigricans.
- Pityriasis rotunda, which is characterized by multiple, round or oval, sharply demarcated scaling patches has been reported in South African black patients with HCC.(27,28)
- Other clinical presentations: The following clinical presentations may be seen in symptomatic patients with HCC:
- Intraperitoneal bleeding due to tumor rupture: Tumor rupture is often associated with sudden onset of severe abdominal pain with distension, an acute drop in the hemoglobin and hypotension, and is most commonly diagnosed by abdominal imaging. Computed tomography (CT) of the abdomen typically demonstrates a liver mass and free intraperitoneal blood.(29) This is a life-threatening complication, and control of bleeding may require emergent angiography and embolization of the bleeding vessel, or surgery.(30) Although the risk of peritoneal dissemination is high, delayed resection may be considered, if feasible.
- Obstructive jaundice caused by invasion of the biliary tree, compression of the intrahepatic duct, or rarely, as a result of hemobilia.
- Fever developing in association with central tumor necrosis.
- Pyogenic liver abscess (very rare).(31)
Extrahepatic metastases: Extrahepatic metastases are present at the time of diagnosis in approximately 10 to 15 percent of cases,(32-34) and they are more common in patients with advanced stage primary tumors (>5 cm, large vessel vascular invasion).(33,35,36) The most common sites of extrahepatic metastases are lung, intra-abdominal lymph nodes, bone,(37) and adrenal gland, in that order. Brain metastases are rare overall (0.2 to 2 percent), although a higher rate has been reported in patients who have already developed metastases elsewhere or with locally advanced HCC.(38,39)
Serum markers
Alpha-fetoprotein: The most commonly used marker for HCC is serum alpha-fetoprotein (AFP) concentration. AFP is a glycoprotein that is normally produced during gestation by the fetal liver and yolk sac, and the serum concentration can be elevated in patients with HCC.
The role of AFP in the diagnostic strategy for HCC is evolving as the accuracy of imaging improves. Serum levels of AFP are typically higher for advanced HCC compared to early HCC, but overall, levels do not correlate well with clinical features of HCC, such as tumor size or vascular invasion. Not all tumors secrete AFP, and serum concentrations are normal in up to 40 percent of small HCC.(40)
An elevated AFP level in conjunction with suspicious but not diagnostic imaging results can have a positive predictive value in the absence of biopsy.(41-43) In addition, an AFP level >1000 ng/mL might exclude potential transplant candidates from being eligible for a standardized MELD exception.
MicroRNAs: Plasma microRNA expression has also been studied as a possible marker of HCC.(44-46) One study examined 934 participants who were healthy, had chronic hepatitis B virus (HBV), had cirrhosis, or had HBV-related HCC [54]. A microRNA panel that included miR-122, miR-192, miR-21, miR-223, miR-26a, and miR-801 accurately identified patients with HCC, regardless of the stage of HCC (AUC 0.89 with a sensitivity of 82 percent and a specificity of 84 percent for the validation set). The panel also accurately differentiated patients with HCC from those who were healthy, had chronic HBV, or had cirrhosis.
Other serum markers: Several serum markers of HCC used alone or in combination with the serum AFP have been evaluated for diagnosis in patients with HCC including des-gamma-carboxy prothrombin,(47,48) lens culinaris agglutinin-reactive AFP (AFP-L3),(49-52) and glypican-3 (a cell-surface heparan sulfate proteoglycan).(53) Studies examining combinations of tumor-specific circulating proteins and mutations in cell-free DNA in the blood show promise for early detection of surgically resectable cancers, although additional data are needed in high-risk cohorts, especially those with cirrhosis.(54) None of these markers can yet be recommended for the diagnosis of HCC.
SCREENING
The screening guidelines from three major liver disease organizations can be aggregated as outlined below.
AASLD
- «Patients at high risk for developing hepatocellular carcinoma should be entered in the surveillance programs.»
- «Patients on the transplant waiting list should be screened for hepatocellular carcinoma”
- «Surveillance for hepatocellular carcinoma should be performed using ultrasonography.»
- «Patients should be screened at 6-month intervals.»(55)
EASL-EORTC
- «A shorter follow-up interval (every 3-4 months) is recommended in the following cases: Where a nodule of less than 1 cm has been detected (see recall policy) and in the follow-up strategy after resection or loco-regional therapies.»
- Recall policy
- «In cirrhotic patients, nodules less than 1 cm in diameter detected by ultrasound should be followed every 4 months the first year and with regular checking every 6 months thereafter.»
- «In cirrhotic patients, diagnosis of hepatocellular carcinoma for nodules of 1-2 cm in diameter should be based on noninvasive criteria or biopsy-proven pathological confirmation.»
- «In cirrhotic patients, nodules more than 2 cm in diameter can be diagnosed for hepatocellular carcinoma based on typical features on one imaging technique. In case of uncertainty or atypical radiologic findings, diagnosis should be confirmed by biopsy.”(56)
DIAGNOSTIC TESTS
Laboratory Studies
Because the outcome in patients with advanced HCC is uniformly dismal, early diagnosis is crucial in order to provide effective treatment. Early diagnosis of HCC is generally the result of routine screening protocols in high-risk patients, including patients with cirrhosis due to viral hepatitis (i.e. from hepatitis B virus [HBV] or hepatitis C virus [HCV]), patients with hemochromatosis, patients with α1-antitrypsin deficiency, or patients who abuse alcohol.
Among patients with cirrhosis, current recommendations include cross-sectional imaging studies every 6-12 months and serum AFP measurements. With aggressive screening, the rate of resectable HCC diagnosed in patients who are at high risk reaches 30-50%, which is nearly twice the rate of unscreened populations.(59) Despite the significant risk of recurrence, even in treated patients, the screening protocols appear to be cost effective in this population.(60)
By virtue of its low cost and morbidity, serum AFP would appear to be an attractive option for screening; unfortunately, it is only 40-64% sensitive because many tumors do not produce AFP at all or do so only at a very advanced stage. AFP levels can be subject to misinterpretation.
AFP is principally the result of production by the tumor or by regenerating hepatocytes. Therefore, AFP levels are also frequently elevated in chronic active hepatitis C (levels of 200-300 ng/mL are not uncommon), but they tend to fluctuate and do not progressively increase. AFP levels can also be elevated because of other conditions, such as following liver resection (transient until regeneration complete), recovery following toxic injury, or seroconversion following hepatitis B infection (typically inducing transient exacerbation of inflammation).
When elevated, the AFP is 75-91% specific and values greater than 400 ng/mL are generally considered diagnostic of HCC in the proper clinical context, including appropriate radiologic findings.(61)
Table 2. Serum Alpha-Fetoprotein (AFP) Determination in Liver Disease(61)
Alpha-fetoprotein (ng/mL)
Interpretation
- HCC likely if accompanied by space-occupying solid lesion(s) in cirrhotic liver or levels are rapidly increasing.
- Diffusely growing HCC, may be difficult to detect on imaging.
- Occasionally in patients with active liver disease (particularly HBV or HCV infection) reflecting inflammation, regeneration, or seroconversion
- Frequent: Regeneration/inflammation (usually in patients with elevated transaminases and HCV) – Regeneration after partial hepatectomy
- If a space-occupying lesion and transaminases are normal, suspicious for HCC
Does not exclude HCC (cirrhotic and noncirrhotic liver)
Laboratory results suggestive or indicative of disease severity include the following:
- Anemia – Low hemoglobin may be related to bleeding from varices or other sources
- Thrombocytopenia – A platelet count below 100,000/μL is highly suggestive of significant portal hypertension/splenomegaly
- Hyponatremia is commonly found in patients with cirrhosis and ascites and may be a marker of advanced liver disease
- Increased serum creatinine level may reflect intrinsic renal disease or hepatorenal syndrome
- Prolonged prothrombin time (PT)/INR reflects significant impairment of hepatic function that may preclude resection
- Elevated liver enzymes reflect active hepatitis due to viral infection, current alcohol use, or other causes
- Increased bilirubin level usually indicates advanced liver disease
- Hypoglycemia may represent end-stage liver disease (no glycogen stores)
Laboratory findings associated with particular disease etiologies include the following:
- Hepatitis B surface antigen (HBsAg)/hepatitis B core antibody (anti-HBc), anti-HCV – Viral hepatitis (current/past)
- Increased iron saturation (>50%) – Underlying hemochromatosis
- Low α1-antitrypsin levels – α1-Antitrypsin deficiency
- Tumor/paraneoplastic phenomena
- Increased AFP – Levels higher than 400 ng/mL are considered diagnostic with appropriate imaging studies
- Hypercalcemia – Ectopic parathyroid hormone production is possible in 5-10% of patients with HCC
- Thrombocytosis (normal/rapid increase in platelet count in patients with a history of thrombocytopenia)
Imaging Studies
Ultrasonography: Accurate diagnosis and surgical planning require adequate cross-sectional imaging studies. Although ultrasonography is commonly used for screening, it does not provide sufficient anatomic detail for planning surgical resection or ablation. Recently, correlation between ultrasonographic findings and explant liver pathology revealed that a significant number of small lesions may not be detected using ultrasound screening. Pooled estimates from one meta-analysis suggested that ultrasonography is only 60% sensitive.(62)
Ultrasonographic identification of HCC can be difficult in the background of regenerative nodules in the cirrhotic liver. In general, HCC appears to be a round or oval mass with sharp, smooth boundaries. The lesions have a range of echogenicity, from hypoechoic to hyperechoic, depending on the surrounding parenchyma and the degree of fatty infiltration. The border between the HCC and the liver can become indistinct with nodular hepatocellular carcinoma. The use of Doppler analysis to characterize the lesion can be helpful, in that HCC is more likely to have a significant arterial blood supply and neovascularization as compared to regenerative nodules.
Computed tomography: Triple-phase CT (including an arterial phase, a portal venous phase, and a late washout phase) has been found to be highly accurate in the diagnosis and characterization of HCCs but, like ultrasonography, may miss smaller lesions. Pooled estimates reveal a sensitivity of 68% and a specificity of 93%.(62) Disadvantages of CT include cost, radiation exposure, and the need for iodinated contrast.
Classic CT findings of HCC include a hypervascular pattern with arterial enhancement and rapid washout during the portal venous phase.(63) In contrast, regenerative nodules generally appear isoattenuating or hypoattenuating when compared to the remaining parenchyma. Other characteristics that support the diagnosis of HCC include visualization of a tumor capsule, demonstration of an internal mosaic resulting from variable attenuation within the tumor, and portal vein branch invasion. Unfortunately, all of these characteristics are more easily demonstrated in large lesions. Consequently, small lesions are frequently missed on CT examination.
Magnetic resonance imaging: MRI provides an excellent method to characterize HCC without radiation and the need for iodinated contrast. Technological improvements have reduced scanning time and improved the specificity of the study. Pooled analysis demonstrated a sensitivity of 81% and a specificity of 85%.(62)
HCC demonstrates a variety of features on MRI depending upon the tumor architecture, grade, and amount of intratumoral fat and glycogen.(63) The lesion ranges from isointense to hyperintense (bright) on T1-weighted images. Similarly, T2 images may vary from isointense to hyperintense. Well-differentiated tumors are more commonly hyperintense on T1 images and isointense on T2 images, whereas moderately or poorly differentiated tumors tend to be hyperintense on T2 images and isointense on T1 images. Although imaging characteristics may be suggestive, a significant overlap may occur between the tumor and regenerative nodules.
The benefits of contrast-enhanced studies must be balanced against the risks if any anatomic or functional renal impairment is possible. Iodinated contrast for CT may worsen renal failure, and gadolinium enhancement on MRI has been linked to a syndrome of severe systemic fibrosis in a patient with renal failure.(64)
Biopsy
The decision to biopsy a lesion suspected of being hepatocellular carcinoma is the subject of ongoing controversy. In patients with large tumors who are not candidates for resection or transplantation, biopsy is frequently not indicated to confirm the diagnosis prior to initiating palliative procedures, because clinical and imaging evidence is convincing and biopsy is potentially risky.
In patients with lesions smaller than 1 cm, fewer than 50% of the lesions will be malignant, and the false-negative result rate is high. Thus, conservative management with close follow-up and no biopsy is recommended.(65)
In patients with 1- to 2-cm lesions, a biopsy should be performed; these patients have a significant risk of malignancy. If the result is positive, they are candidates for resection, transplantation, or ablative therapy. As in the smaller lesions, there is a significant false-negative result rate, and close follow-up is indicated in patients with a negative biopsy result.
Patients with lesions larger than 2 cm, cirrhosis, characteristic imaging studies, and elevated AFP values can be managed without biopsy. In these patients, the risk of tumor seeding must be taken into account. Whereas some groups require biopsy before transplantation,(65) others are willing to proceed on clinical characteristics alone.(66) In patients with more atypical findings on imaging studies, the value of AFP should not be overemphasized, because an excessive number of patients submitted to transplantation did not have HCC.(67)
In patients with cirrhosis who are being considered for resection, survival following resection has been previously correlated with the degree of portal hypertension. In some centers, determination of the wedged hepatic vein pressure is advocated to then determine the safety of resection. Resection can, in general, be safely undertaken in patients with a wedged hepatic venous pressure gradient of less than 10.(65) Patients should also have a platelet count lower than 100,000/μL and a normal bilirubin level. In patients with small tumors but significant hepatic dysfunction, transplantation is the preferred option.
Staging
The prognosis of HCC is a reflection of both tumor characteristics (i.e. size, location, tumor biology) and the degree of underlying liver disease. The traditional pathological TNM (tumor-node-metastasis) staging system, while helpful in determining a prognosis in patients undergoing resection, is not as useful in planning treatment, because it fails to include measures of the severity of the liver disease. However, the tumor size is predictive of outcome, as it predicts the likelihood of major venous involvement.(68)
Likewise, the Child-Pugh-Turcotte score predicts perioperative survival after resection, but it does not incorporate tumor size, number, and location, which have important implications for respectability and treatment. Among the scales that integrate the tumor and liver disease characteristics, the Barcelona Clinic Liver Cancer (BCLC) system (see the image below),(65) the Japan Integrated Staging System, and the Cancer of the Liver Italian Program (CLIP) are the most widely used staging systems.
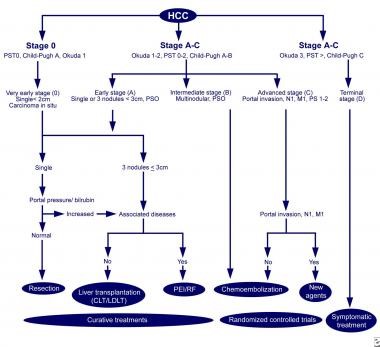
The Barcelona-Clinic Liver Cancer (BCLC) approach to hepatocellular carcinoma management.(69)
The BCLC system is very useful in deciding among potential treatment options and correlates best with patient outcome among the major staging systems.(70)
In the BCLC system, stage 0 patients have lesions smaller than 2 cm, normal bilirubin levels, and normal portal pressure measurements. These patients can often undergo resection safely with excellent long-term survival.
Patients with larger tumors (i.e. single tumors < 5 cm or multiple [≤3] tumors < 3 cm) are considered for resection if they have preserved liver function or for transplantation if they have decompensated cirrhosis.
In patients whose tumor exceeds these measurements, palliative therapy can be offered depending upon hepatic reserve, but long-term survival (>3 years) is achieved by fewer than 10% of patients.
TREATMENT OPTIONS
Hepatocellular carcinoma (HCC) is an aggressive tumor that often occurs in the setting of chronic liver disease and cirrhosis. It is typically diagnosed late in its course, and the median survival following diagnosis is approximately 6 to 20 months.(72) Although the mainstay of therapy is surgical resection, the majority of patients are not eligible because of tumor extent or underlying liver dysfunction.
Several other treatment modalities are available, including:
- Liver transplantation
- Radiofrequency ablation, microwave ablation, and cryoablation
- Percutaneous ethanol or acetic acid ablation
- Irreversible electroporation
- Transarterial chemoembolization (TACE) and bland embolization
- Transarterial radioembolization (TARE, also called radioembolization)
- Radiation therapy and stereotactic radiation therapy
- Systemic chemotherapy, with cytotoxic agents and molecularly targeted therapies
- Immunotherapy
Options are determined both by disease extent and the severity of underlying liver disease, which limits the tolerance to any therapy (medical, interventional, or surgical). For patients with cirrhosis, the Child-Turcotte-Pugh classification is used most widely to stratify patients according to their underlying liver disease, although other measures are increasingly used.
Importance of multidisciplinary care:
The wide variety of treatments for hepatocellular carcinoma (HCC) are offered by different specialties: surgery, radiation oncology, medical oncology, and interventional radiology. Multidisciplinary evaluation and planning, frequently overseen by hepatologists, typically result in more thoroughly vetted recommendations and are less likely to result in a recommendation for a procedure for which a single provider has expertise but that may not represent the optimal therapy for an individual patient.
The majority of patients with HCC have underlying liver disease. Patients who undergo any form of therapy for HCC are at high risk for progression to liver failure because of their underlying liver disease, and proper monitoring, assessment, and treatment of the underlying liver disease may have a major impact on long-term survival. The comprehensive care of patients with cirrhosis includes antiviral therapy for hepatitis B and C virus, immunization against hepatitis A and B virus (if indicated), regular surveillance for HCC with abdominal imaging, and endoscopic screening and surveillance for varices.
Potentially resectable disease:
The preferred therapy for localized hepatocellular carcinoma (HCC) is surgical resection, but the majority of patients are not eligible because of tumor extent or underlying liver dysfunction.
Potentially curative partial hepatectomy is the optimal treatment for HCC in patients with adequate liver functional reserve (i.e. no worse than Child-Turcotte-Pugh A cirrhosis). The ideal patient for resection has a solitary HCC confined to the liver that shows no radiographic evidence of invasion of the hepatic vasculature, no evidence of portal hypertension, and well-preserved hepatic function. Long-term relapse-free survival rates average 40 percent or better and five-year survival rates as high as 90 percent are reported in carefully selected patients.
Preoperative evaluation should focus on the likelihood of disease being confined to the liver and whether the anatomic constraints of the intrahepatic tumor and the underlying liver function will permit resection. Although many surgeons restrict eligibility for resection to patients with tumors that are ≤5 cm in diameter, there is no general rule regarding tumor size for selection of patients for resection. Patients with a solitary HCC without vascular invasion have a similar survival probability regardless of tumor size, although patients with smaller tumors tend to have a better outcome.
According to the current American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging system for HCC, most consider stage IIIB, IVA, or IVB disease to be incurable by resection. These stages are defined by invasion of a major portal or hepatic vein, direct invasion of organs other than the gallbladder, perforation of the visceral peritoneum, and nodal as well as distant metastases. However, hepatic resection for stage IIIB, IIIC, and IVA disease may be considered for very rare and selected cases, and only in a center of excellence. Clinical benefits and long-term survival can be achieved in only a very small minority of patients.
Although the rare situation of tumor rupture of an HCC suggests a high likelihood of peritoneal seeding and a poor outcome from resection, this is not inevitable. Several retrospective series suggest a low but defined long-term survival rate following resection in such situations.(73) The most common approach is initial control of bleeding (which may require embolization or emergency surgery) followed by a formal staging evaluation and a subsequent attempt at resection, if feasible.
Assessment of hepatic reserve is paramount to selection for resection. Perioperative mortality is twice as high in cirrhotic as in noncirrhotic patients, unless proper patient selection is applied. For patients with cirrhosis, surgical resection is most safely performed in those with Child-Turcotte-Pugh class A disease who have a normal bilirubin and well-preserved liver function. Patients with Child-Turcotte-Pugh class A liver function who require major resection should undergo liver volumetry, and portal vein embolization (PVE) should be considered if liver volume is insufficient.
Resection as an option for patients with HCC and Child-Turcotte-Pugh B cirrhosis is associated with increased morbidity and mortality, so it remains controversial. Transplantation may represent a better choice if the patient fulfills transplant criteria.
Preoperative PVE is a valuable adjunct to major liver resection to increase the future liver remnant, particularly for right-sided tumors. Transarterial chemoembolization (TACE) may be a complementary procedure prior to PVE in such patients as it eliminates the arterial blood supply to the tumor and also embolizes potential arterioportal shunts that attenuate the effects of PVE in cirrhotic livers.
Role of adjuvant therapy: Adjuvant antiviral therapy improves outcomes after potentially curative treatment of hepatitis B virus (HBV) related HCC and is recommended for those with active viral infection. There is also some evidence of benefit for adjuvant TACE, although this approach is used predominantly in China. The benefit of any other form of adjuvant therapy following potentially curative resection of HCC remains unclear and cannot be recommended.
Unresectable disease:
For patients who are not candidates for resection either because of the extent of their HCC or poor underlying liver function,(74) options include liver transplantation, locoregional hepatic therapies such as thermal ablation, embolization, and radiation therapy (RT), and systemic therapy.
Liver transplantation: For patients who are not surgically resectable, liver transplantation is the only other potentially curative option. Virtually all patients who are considered for liver transplantation are unresectable because of the degree of underlying liver dysfunction rather than tumor extent. Orthotopic liver transplantation (OLT) is a suitable option for patients with liver disease (usually cirrhosis) who would not tolerate liver resection.
A major disadvantage with OLT (in addition to the need for lifelong immunosuppression, with its attendant risks) is the long waiting time for donor organs. Even with higher priority MELD scores, waiting times for a donor organ may be many months. Bridging therapy with TACE, radiofrequency ablation (RFA), or partial hepatectomy is a reasonable approach while a patient is on the waiting list for OLT in order to maintain his or her eligibility. Living donor transplantation is being applied, although this approach is also controversial. Waiting time is obviously much shorter, and results seem to be at least as good as with deceased donor livers.
Localized disease and ineligible for resection or transplant, no macrovascular invasion: Options for patients with disease that is limited to the liver but who are not candidates for resection or transplantation include liver-directed approaches (e.g. RFA, microwave ablation, embolization, external beam RT) and systemic therapy.
Selection of therapy: The selection of treatment is determined by the severity of underlying liver disease, the size and distribution of the intrahepatic tumors, the vascular supply, and the patient’s overall performance status.
Locoregional forms of therapy, such as TACE or radioembolization, may be preferable to systemic therapy alone for initial treatment of patients with locally advanced unresectable HCC without extrahepatic metastases, as long as they are suitable candidates.
In general:
- The best results with RFA and microwave ablation are in patients with one or two tumors <4 cm in diameter. For cirrhotic patients, most clinicians restrict these approaches to those with Child-Turcotte-Pugh class A or B severity only. RFA and microwave ablation have also been used as «bridging» therapy in patients awaiting liver transplantation to reduce the rate of dropout because of tumor progression.
- TACE is used most often for the treatment of large unresectable HCCs that are not amenable to other treatments, such as resection or RFA; the best candidates are patients with unresectable HCC without vascular invasion or extrahepatic spread, and with preserved liver function (i.e. Child-Turcotte-Pugh A or B cirrhosis). In addition, the use of TACE as «bridging therapy» prior to liver transplantation for HCC is common.
The available data do not support benefit from combining TACE with either RFA or sorafenib.
- Radioembolization using intraarterial injection of yttrium-90 (90-Y)-labeled microspheres induces extensive tumor necrosis with an acceptable safety profile. However, there are no studies demonstrating an impact on survival and no adequately powered randomized trials comparing TACE with radioembolization.(12) As a result, there is no consensus as to the optimal use of this therapy, particularly when and if it should be chosen over TACE for treatment of unresectable HCC.
- One clinical scenario in which radioembolization may be preferred over TACE is in the setting of an HCC complicated by malignant main or lobar portal vein thrombus. Other potential treatment options in this setting include stereotactic RT, TACE plus RT, or where available, proton beam irradiation. In general, survival is short, and whether any form of locoregional therapy benefits these patients above and beyond what might be accomplished with systemic therapy is unclear.
- For patients with liver-isolated HCC who are eligible for liver-directed nonsurgical therapies, two relevant questions are whether the addition of systemic therapy improves results compared with locoregional therapy alone, and whether initial systemic therapy provides better outcomes than can be achieved with initial liver-directed therapy. Taken together, the available evidence from limited randomized trials does not support a clear benefit for the addition of sorafenib to TACE and also suggests that embolization outperforms initial sorafenib in terms of efficacy and adverse event profile.
Ablation:
Radiofrequency ablation: RFA involves the local application of radiofrequency thermal energy to the lesion; high-frequency alternating current moves from the tip of an electrode into the tissue surrounding that electrode. As the ions within the tissue attempt to follow the change in direction of the alternating current, their movement results in frictional heating of the tissue. As the temperature within the tissue becomes elevated beyond 60ºC, cells begin to die, resulting in a region of necrosis surrounding the electrode.
RFA is a reasonable option for patients who do not meet resectability criteria for HCC and yet are candidates for a liver-directed procedure based upon the presence of liver-only disease. Although there is no absolute tumor size beyond which RFA should not be considered, the best outcomes are in patients with a single tumor <4 cm in diameter. For cirrhotic patients, some clinicians restrict RFA to those with Child-Turcotte-Pugh class A or B severity only. As noted above, RFA has also been used as «bridging» therapy in patients awaiting liver transplantation to reduce the rate of dropout because of tumor progression. RFA may not be feasible due to anatomic considerations (e.g. dome of the liver, near the gallbladder, lesions near blood vessels due to heat sink effect).
Microwave ablation: Microwave ablation, in which an implanted electrode delivers a high-frequency microwave into the tumor tissue, has been used for hepatic tumor ablation for years in China and Japan. One advantage of microwave ablation over RFA is the ability to perform multiple ablations simultaneously; there is no comparable RFA system with the capacity to drive multiple electrically dependent electrodes. Although there is less experience with this approach than with RFA, the available data suggest that results are at least comparable with those achieved with RFA, and microwave ablation has replaced RFA at some institutions.
Percutaneous ethanol or acetic acid ablation: As with RFA, percutaneous ethanol injection may be considered for patients with small HCCs who are not candidates for resection due to their poor functional hepatic reserve. Before the advent of RFA, percutaneous ethanol injection was the most widely accepted minimally invasive method for treating such patients. Although it is low cost, requires a minimal amount of equipment, and has good clinical results, the greater efficacy of RFA has supplanted its use in many institutions.
Similar results have been achieved with the percutaneous injection of acetic acid, which may have fewer side effects than ethanol.
Cryoablation: Cryoablation, the application of alternating freeze-thaw cycles through the use of a cryoprobe inserted directly into the tumor, has been most frequently applied in patients who are determined to have unresectable HCC intraoperatively. It may be more appropriate than RFA for situations in which there is a high likelihood of collateral thermal damage or in which the lesion is very peripheral.
Embolization:
The observation that the majority of the blood supply to HCCs is derived from the hepatic artery has led to the development of techniques designed to eliminate the tumor’s blood supply by particle embolization alone (bland embolization), particle embolization coupled with the administration of cytotoxic chemotherapy, with or without lipiodol, into the hepatic artery (TACE), or embolization with particles that are embedded with radioactive isotopes (e.g. 90-Y-tagged glass microspheres; radioembolization, also called selective internal RT).
TACE versus bland transarterial embolization: Bland particle transarterial embolization, which relies solely on induction of tumor ischemia through disruption of the blood supply to the tumor, has been utilized successfully for the treatment of both unresectable and recurrent HCC. However, meta-analyses have come to different conclusions, with one suggesting that a survival benefit relative to best supportive care could be shown for TACE but not for transarterial embolization,(76) and the other two failing to find firm evidence to support or refute TACE over transarterial embolization for patients with unresectable HCC.(77,78)
Nevertheless, at least two trials demonstrate a survival benefit for TACE in this setting,(79,80) and the majority of published experience is with TACE. As a result, many current international guidelines recommend TACE over bland transarterial embolization for unresectable HCC(81-83), although the National Comprehensive Cancer Network (NCCN) guidelines do not state a preference.
Conventional TACE involves the injection of a chemotherapeutic agent, with or without lipiodol or a procoagulant material, into the hepatic artery. Lipiodol is an oily contrast agent that promotes intratumoral retention of chemotherapy drugs. There is tremendous heterogeneity in the TACE techniques and schedules used in worldwide clinical practice, which hinders the comparison of results from different studies and complicates the conduction of high-quality multicenter TACE trials.
The most recent and more common method of TACE involves the use of drug-eluting polyvinyl alcohol microspheres («beads»), which seem to cause less toxicity than lipiodol but with similar efficacy. Simultaneous or sequential occlusion of the hepatic artery until stagnation of blood flow to the tumor occurs may result in greater antitumor efficacy than chemotherapy alone.
TACE is used most often for the treatment of large unresectable HCCs that are not amenable to other treatments, such as resection or RFA; the best candidates are patients with unresectable HCC without vascular invasion or extrahepatic spread, and with preserved liver function (i.e. Child-Turcotte-Pugh A or B [score <8] cirrhosis). In addition, the use of TACE as «bridging therapy» prior to liver transplantation for HCC is common.
Although most patients who have potentially resectable HCC are not treated with TACE prior to resection, patients with large right-sided tumors who will need right hepatectomy may benefit from TACE combined with PVE prior to the planned resection.
Absolute contraindications to TACE include absent or severely reduced portal vein flow (e.g. tumoral or nontumoral portal vein occlusion or hepatofugal blood flow) and decompensated cirrhosis (Child-Turcotte-Pugh B [score >8] including jaundice, uncontrolled hepatic encephalopathy, refractory ascites, and/or hepatorenal syndrome [especially type I]).(84,85)
TACE plus local treatments: Combining local ablation treatment (e.g. RFA, microwave ablation, or external beam RT) and TACE can theoretically overcome the limitations of either TACE or ablation when used alone:
Radioembolization: Radioembolization using intraarterial injection of 90-Y-labeled microspheres induces extensive tumor necrosis with an acceptable safety profile. However, there are no studies demonstrating an impact on survival and no consensus as to the optimal use of this therapy, particularly when and if it should be chosen over TACE for treatment of unresectable HCC.
Selection for radioembolization continues to require evaluation by a multidisciplinary team. Benefits over other forms of local therapy include relatively low toxicity, the potential to treat patients with significant tumor burden (often in a single setting, rather than multiple sessions, as with classic TACE), and relatively limited side effects. However, high cost, certain anatomical constraints (e.g. passthrough of the radioactive material to the lung in some patients with shunting), and a question of less effective tumor necrosis than seen with TACE limit the utility of this treatment. One clinical scenario in which radioembolization may be preferred over TACE is in the setting of an HCC complicated by malignant branch or lobar portal vein thrombus.
Role for systemic therapy:
There has been a resurgence of interest and enthusiasm for systemic therapy of HCC with the emergence of data showing that the molecularly targeted agents sorafenib and regorafenib improve survival compared with best supportive care alone; a survival benefit has also been shown in the second-line setting for nivolumab, an immune checkpoint inhibitor, and lenvatinib has demonstrated noninferiority to first-line sorafenib.
For patients with liver-isolated HCC who are eligible for liver-directed nonsurgical therapies, two relevant questions are whether the addition of systemic therapy improves results compared with locoregional therapy alone, and whether initial systemic therapy provides better outcomes than can be achieved with initial liver-directed therapy. Taken together, the available evidence from limited randomized trials does not support a clear benefit for the addition of sorafenib to TACE and also suggests that embolization outperforms initial sorafenib in terms of efficacy and adverse event profile.
TACE plus sorafenib: Sorafenib is a multikinase inhibitor acting on the vascular endothelial growth factor receptor (VEGFR), among others. It has been hypothesized that administering sorafenib might be useful to target upregulation of TACE-induced angiogenic factors and, therefore, improve outcomes from TACE treatment.
Radioembolization versus sorafenib: Whether results with radioembolization are better than those that can be achieved with systemic administration of sorafenib was directly addressed in two different multicenter trials, both of which concluded that radioembolization was associated with a higher objective tumor response rate, similar median survival, and fewer adverse events.(86,87) Although radioembolization did not result in superior survival compared with sorafenib in either trial, given the more favorable adverse event profile, these results support the general principle that locoregional forms of therapy, such as radioembolization, are preferable to systemic therapy alone for initial treatment of patients with locally advanced unresectable HCC without extrahepatic metastases, as long as they are suitable candidates for liver-directed therapy.
TACE versus sorafenib: Trial that tested whether the combination of TACE plus external beam RT was better than initial sorafenib in patients with liver-isolated HCC with invasion of the main or a first or second branch portal vein and preserved unilateral portal blood flow also concluded that first-line treatment with TACE plus RT was well tolerated and provided improved progression-free survival, objective response rate, time to progression, and overall survival compared with sorafenib treatment.
Patients with macroscopic vascular invasion:
For patients with tumor invasion, occlusion, or thrombus involving the main portal vein or a major branch, options include radioembolization, stereotactic RT, TACE plus RT, proton beam irradiation (where available), and initial systemic therapy. In general, survival is short, and patients are prone to rapid disease progression when treated with initial sorafenib.(88-90) Based upon a single trial that demonstrates that TACE plus RT provides outcomes that are better than those that can be achieved with sorafenib, TACE plus RT is a reasonable option for patients with tumor invasion of a first or second branch of the portal vein as long as unilateral portal blood flow is preserved and liver function is well preserved (Child-Turcotte-Pugh A). For patients with tumor thrombus involving the main portal vein or a major branch with no portal vein flow, reversal of portal vein flow, or more severe degrees of liver dysfunction, other options are more appropriate, including RT (transarterial radioembolization or external beam RT) in some patients.
Stereotactic body radiation therapy: HCC is a radiosensitive tumor, but it is located in an extremely radiosensitive organ. As a whole, the liver can only tolerate approximately 20 Gy. With the development of three-dimensional conformal radiation therapy (3D-CRT) techniques, RT can be more safely delivered to the tumor-bearing parts of the liver with less liver toxicity. Technologic developments in delivering more precisely targeted RT (intensity-modulated RT and image-guided approaches, including stereotactic body RT) appear to further improve the risk-to-benefit ratio, although they do not alter the high recurrence rates in other nontreated areas of the liver.
The most promising of these approaches is stereotactic body RT, a technique in which a single (sometimes called stereotactic radiosurgery) or a limited number of high-dose radiation fractions are delivered to a small, precisely defined target using multiple, nonparallel radiation beams. The beams converge precisely on the target lesion, minimizing radiation exposure to adjacent normal tissue. This targeting allows treatment of extracranial sites in either a single or a limited number of dose fractions.
TACE plus radiation therapy: The only trial that has addressed the relative benefit of local liver-directed therapy versus systemic therapy for patients with macroscopic vascular invasion compared TACE plus external beam RT (within three weeks of the first TACE, 45 Gy using 3D-CRT planning with a fraction size of 2.5 to 3 Gy) with initial sorafenib (400 mg twice daily) in 90 treatment-naive patients with liver-confined HCC invading at least the first or second branch of the portal vein and with preserved unilateral portal blood flow.(91) All patients had Child-Turcotte-Pugh A liver function, and 85 percent had HBV associated HCC. The TACE procedure was repeated every 6 of the first 24 weeks and every six to eight weeks thereafter. The group receiving TACE plus RT had significantly higher progression-free survival rates at 12 weeks (the main endpoint, 87 versus 34 percent), higher radiographic response rates at 24 weeks, significantly longer median time to progression, and significantly greater median overall survival (55 versus 43 weeks).
Radioembolization: While main portal vein thrombus has historically been considered a contraindication to transarterial embolization, collective experience in over 200 patients suggests the safety and efficacy of radioembolization. There is theoretically less arterial ischemia induced by radioembolization than chemoembolization because of the smaller particle size (32 versus 70 to 300 microns with drug-eluting beads), so the portal blood supply is less important from the standpoint of toxicity. Although radioembolization appears to be safe in these patients, median survival is short in many series, particularly in those with Child-Turcotte-Pugh B cirrhosis, so the benefit of this approach remains uncertain.
Proton beam irradiation: There is a growing body of evidence, primarily from Japan, supporting the use of proton beam irradiation, particularly for patients with large tumors or portal vein thrombus. However, unresolved issues include whether these outcomes could be achieved with other approaches (including modern TACE with RT, or stereotactic body RT) and which patients, if any, would do better with proton beam therapy. Proton beam irradiation is not widely available.
Treatment at progression:
For patients with radiologic progression after resection or locoregional liver-directed therapy, additional liver-directed therapy might be possible depending upon tumor location and underlying liver function.
Once all forms of locoregional liver-directed therapy are exhausted and for patients who recur following liver transplantation, systemic therapy is an option if performance status and underlying liver function are adequate. Supportive care alone is appropriate for patients with Child-Turcotte-Pugh C cirrhosis and for those with a poor functional status or extensive comorbidity.
The definition of «failure of local therapy» is evolving. The rapidly changing availability of potentially effective and survival-prolonging systemic therapies in HCC has enabled clinicians to offer alternatives to patients who have locally progressive intrahepatic disease, even if they may be eligible for additional liver-directed therapy. Specifically, the acceptance of the toxicity of a risky local procedure in a patient with borderline indications may be tempered by having viable alternative systemic options that did not previously exist.
Patients ineligible for resection, transplantation, and local therapies
Systemic therapy: For patients who are ineligible for liver-directed therapy or for patients demonstrating progression on locoregional therapy, systemic therapy is an option if performance status and underlying liver function are adequate.
Until 2008, no effective therapy existed for patients with advanced-stage HCC or for those failing local therapies. However, there has been a resurgence of interest and enthusiasm for systemic therapy of HCC with the emergence of data showing that the molecularly targeted agents sorafenib and regorafenib improve survival compared with best supportive care alone. Subsequently, a survival benefit has also been shown in the second-line setting for nivolumab, an immune checkpoint inhibitor, and lenvatinib has demonstrated noninferiority to first-line sorafenib. These results have radically changed the treatment landscape for advanced HCC.
Initially reported in 2007, the multinational randomized SHARP trial demonstrated a modest but statistically significant survival benefit for sorafenib (a multitargeted tyrosine kinase inhibitor) over supportive care alone in patients with advanced HCC. A survival benefit was also demonstrated in Asian patients. These results established sorafenib monotherapy as a new reference standard systemic treatment for advanced HCC and formed the basis for approval of sorafenib for first-line treatment of unresectable HCC in the United States.
However, the generalizability of the survival benefits seen in this and one other subsequent randomized trial of sorafenib has been questioned, in particular whether benefit differs according to the etiology of the HCC. Benefit from sorafenib may be much higher in patients with HCC related to hepatitis C virus (HCV) infection than in those with other underlying risk factors. However, sorafenib was shown to be beneficial in an HBV predominant population in the Asia-Pacific trial.
However, whether patients with other (nonviral) etiologies of chronic liver disease should be offered a first-line treatment other than sorafenib (e.g. lenvatinib) is unclear; there are no prospective studies that have examined treatment response to any other form of molecularly targeted therapy based upon HCC etiology.
Lenvatinib is a reasonable alternative treatment, especially for patients who are intolerant of sorafenib.
Second-line therapy is an option for patients whose tumors progress while on first-line therapy and whose performance status and liver function are sufficient to tolerate it. The best regimen is not established, and there are no biomarkers to guide selection of one agent over another. For patients who are not eligible for clinical trials or if they are not available, options include tyrosine kinase inhibitors (i.e. sorafenib [if it was not administered for first-line therapy] or regorafenib) or the immune checkpoint inhibitor nivolumab.
The side effect profile of each individual regimen must be carefully considered in patients who have advanced liver disease and/or a short life expectancy. Lenvatinib is another option for treatment after failure of sorafenib. However, whether lenvatinib will work after failure of sorafenib is not known; there are no trials to inform this issue.
The available data suggest a modest degree of antitumor efficacy for several conventional cytotoxic agents and/or combination drug regimens. However, the appropriate selection of patients for cytotoxic chemotherapy, especially in view of the advances in molecularly targeted therapies and immunotherapy, is not clear. It should be noted that molecularly targeted agents and immune checkpoint inhibitors are expensive. For patients who are unable to obtain these agents, systemic chemotherapy remains an option, although the best regimen is not established.
GUIDELINES
To view, “Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations”, please click on below link:
https://link.springer.com/content/pdf/10.1007%2Fs00268-016-3700-1.pdf
To view, “Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report1”, please click on below link:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3417764/pdf/nihms392425.pdf
To view World journal Gastroenterology guidelines, “Hepatocellular carcinoma (HCC): a global perspective, please click on below link:
http://www.worldgastroenterology.org/UserFiles/file/guidelines/hepatocellular-carcinoma-english-2009.pdf
To review the NCCN guidelines on hepatobiliary cancers, please click on below link:
https://www.nccn.org/patients/guidelines/hepatobiliary/32/
To review, Hepatocellular Carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up”, please click on below link:
https://www.esmo.org/Guidelines/Gastrointestinal-Cancers/Hepatocellular-Carcinoma
CONSULTATION AND LONG TERM MONITORING
Despite optimal treatment, HCC continues to have a high recurrence rate. It recurs in 50-80% of patients following resection, with the majority of recurrences developing within 2 years. Careful follow-up in the postoperative period is mandatory. Early recurrence after resection is associated with a dismal prognosis, reducing 5-year survival rates from 70% to 30%. Factors that increase the likelihood of recurrence include the presence of multiple foci of HCC, liver capsule invasion, and tumor size greater than 5 cm. Vascular invasion, both microscopic and macroscopic, also correlates with a higher risk of recurrence.
Among patients undergoing liver transplantation, the rate of recurrence is dependent upon the characteristics of the tumor in the explanted liver. Overall recurrence in patients transplanted within the Milan criteria is 4-10%. The majority of these recurrences occur early (8-14 months); however, as many as 30% of recurrences may occur late. In these patients, 23% develop intrahepatic-only recurrence, 39% develop both intrahepatic and extrahepatic recurrence, and 39% develop extrahepatic-only recurrence. Common extrahepatic sites of metastatic disease include lung, bone, central nervous system, and adrenal glands.
Resection in the post-transplant population can be accomplished in up to one third of patients. In those patients who undergo successful resection, 4-year survival rates increase from 14% to 57%, justifying an aggressive approach.
Unfortunately, no established guidelines exist regarding the frequency of imaging procedures in the postoperative period. In general, CT should be performed at 1 month post resection to ensure complete tumor clearance. After this initial scan, serum AFP measurements and repeat imaging studies (e.g. ultrasonography, CT, MRI) should be obtained every 3-6 months, depending on the likelihood of recurrence. After 2-3 years, it appears safe to increase the follow-up interval.
PRECAUTIONS
Strategies to limit the epidemic of HCC are likely to pay off in the long term. The vaccination campaign against hepatitis B has already resulted in a reduced incidence of HCC in Taiwan. Moreover, failure to complete HBV vaccination continues to lead to HCC in patients.
Other strategies to reduce the incidence of HCC include the treatment of HBV and HCV infection to eradicate the virus with rapidly effective therapies, including pegylated interferons, nucleoside analogues (HBV), and ribavirin (HCV). Promising protease inhibitors are in ongoing clinical trials, and adequate screening of high-risk patients is needed to treat small lesions early. Analysis of patients from the Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) trial found that in patients with chronic hepatitis C who did not have a sustained virologic response to therapy, long-term pegylated interferon therapy does not reduce the incidence of HCC.
Other preventive approaches include programs to reduce obesity and type 2 diabetes. Major efforts are also needed to specifically warn patients with chronic liver disease to discontinue alcohol abuse. Hemochromatosis should be recognized in a timely manner.
REFERENCES
- Luca Cicalese. Hepatocellular Carcinoma. Medscape. https://emedicine.medscape.com/article/197319-overview#a6. Accessed on November 29 2018
- F. Badar and S. Mahmood, “Hospital-based cancer profile at the Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan,” Journal of the College of Physicians and Surgeons Pakistan, vol. 25, no. 4, pp. 259–263, 2015
- Y. Bhurgri, A. Bhurgri, S. H. Hassan et al., “Cancer incidence in Karachi, Pakistan: first results from Karachi Cancer Registry,” International Journal of Cancer, vol. 85, no. 3, pp. 325–329, 2000.
- Y. Bhurgri, A. Bhurgri, S. Pervez et al., “Cancer profile of hyderabad, Pakistan 1998–2002,” Asian Pacific Journal of Cancer Prevention, vol. 6, no. 4, pp. 474–480, 1998.
- Y. Bhurgri, S. Pervez, N. Kayani et al., “Cancer profile of Larkana, Pakistan (2000–2002),” Asian Pacific Journal of Cancer Prevention, vol. 7, no. 4, pp. 518–521, 2006
- Peter Ferenci. Hepatic encephalopathy: Pathogenesis. Uptodate. https://www.uptodate.com/contents/hepatic-encephalopathy-pathogenesis?search=hepatic%20encephalopathy%20pathophysiology&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Accessed on November 29 2018
- Beasley RP, Hwang LY, Lin CC, et al. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21. 2(8256):1129-33
- Zhang X, Zhang H, Ye L. Effects of hepatitis B virus X protein on the development of liver cancer. J Lab Clin Med. 2006 Feb. 147(2):58-66
- McKillop IH, Moran DM, Jin X, et al. Molecular pathogenesis of hepatocellular carcinoma. J Surg Res. 2006 Nov. 136(1):125-35
- Li M, Zhao H, Zhang X, et al. Inactivating mutations of the chromatin remodeling gene ARID2 in hepatocellular carcinoma. Nat Genet. 2011 Aug 7. 43(9):828-9
- Cameron RG, Greig PD, Farber E, et al. Small encapsulated hepatocellular carcinoma of the liver. Provisional analysis of pathogenetic mechanisms. Cancer. 1993 Nov 1. 72(9):2550-9
- Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev. 2005. 1(3):253-60
- D’Errico A, et al. Histogenesis of primary liver carcinomas: strengths and weaknesses of cytokeratin profile and albumin mRNA detection, Hum. Pathol. , 1996, vol. 27 (pg. 599-604)
- Yuen MF, et al. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience, Hepatology , 2000, vol. 31 (pg. 330-335)
- Trevisani F, et al. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian Experience), Am. J. Gastroenterol. , 2002, vol. 97 (pg. 734-744)
- Kew MC, Dos Santos HA, Sherlock S. Diagnosis of primary cancer of the liver. Br Med J 1971; 4:408
- Luo JC, Hwang SJ, Wu JC, et al. Clinical characteristics and prognosis of hepatocellular carcinoma patients with paraneoplastic syndromes. Hepatogastroenterology 2002; 49:1315
- Eastman RC, Carson RE, Orloff DG, et al. Glucose utilization in a patient with hepatoma and hypoglycemia. Assessment by a positron emission tomography. J Clin Invest 1992; 89:1958.
- Tietge UJ, Schöfl C, Ocran KW, et al. Hepatoma with severe non-islet cell tumor hypoglycemia. Am J Gastroenterol 1998; 93:997
- Sakisaka S, Watanabe M, Tateishi H, et al. Erythropoietin production in hepatocellular carcinoma cells associated with polycythemia: immunohistochemical evidence. Hepatology 1993; 18:1357
- Kew MC, Fisher JW. Serum erythropoietin concentrations in patients with hepatocellular carcinoma. Cancer 1986; 58:2485
- Yen TC, Hwang SJ, Wang CC, et al. Hypercalcemia and parathyroid hormone-related protein in hepatocellular carcinoma. Liver 1993; 13:311
- Bruix J, Castells A, Calvet X, et al. Diarrhea as a presenting symptom of hepatocellular carcinoma. Dig Dis Sci 1990; 35:681.
- Steiner E, Velt P, Gutierrez O, et al. Hepatocellular carcinoma presenting with intractable diarrhea. A radiologic-pathologic correlation. Arch Surg 1986; 121:849
- Gregory B, Ho VC. Cutaneous manifestations of gastrointestinal disorders. Part II. J Am Acad Dermatol 1992; 26:371
- Dogra S, Jindal R. Cutaneous manifestations of common liver diseases. J Clin Exp Hepatol 2011; 1:177
- Berkowitz I, Hodkinson HJ, Kew MC, DiBisceglie AM. Pityriasis rotunda as a cutaneous marker of hepatocellular carcinoma: a comparison with its prevalence in other diseases. Br J Dermatol 1989; 120:545.
- DiBisceglie AM, Hodkinson HJ, Berkowitz I, Kew MC. Pityriasis rotunda. A cutaneous marker of hepatocellular carcinoma in South African blacks. Arch Dermatol 1986; 122:802
- Choi BG, Park SH, Byun JY, et al. The findings of ruptured hepatocellular carcinoma on helical CT. Br J Radiol 2001; 74:142
- Chearanai O, Plengvanit U, Asavanich C, et al. Spontaneous rupture of primary hepatoma: report of 63 cases with particular reference to the pathogenesis and rationale treatment by hepatic artery ligation. Cancer 1983; 51:1532
- Lin YT, Liu CJ, Chen TJ, et al. Pyogenic liver abscess as the initial manifestation of underlying hepatocellular carcinoma. Am J Med 2011; 124:1158
- Uka K, Aikata H, Takaki S, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol 2007; 13:414.
- Yi J, Gwak GY, Sinn DH, et al. Screening for extrahepatic metastases by additional staging modalities is required for hepatocellular carcinoma patients beyond modified UICC stage T1. Hepatogastroenterology 2013; 60:328.
- Jin YJ, Lee HC, Lee D, et al. Role of the routine use of chest computed tomography and bone scan in staging workup of hepatocellular carcinoma. J Hepatol 2012; 56:1324.
- Yoon KT, Kim JK, Kim DY, et al. Role of 18F-fluorodeoxyglucose positron emission tomography in detecting extrahepatic metastasis in pretreatment staging of hepatocellular carcinoma. Oncology 2007; 72 Suppl 1:104.
- Yuki K, Hirohashi S, Sakamoto M, et al. Growth and spread of hepatocellular carcinoma. A review of 240 consecutive autopsy cases. Cancer 1990; 66:2174.
- Harding JJ, Abu-Zeinah G, Chou JF, et al. Frequency, Morbidity, and Mortality of Bone Metastases in Advanced Hepatocellular Carcinoma. J Natl Compr Canc Netw 2018; 16:50
- Choi HJ, Cho BC, Sohn JH, et al. Brain metastases from hepatocellular carcinoma: prognostic factors and outcome: brain metastasis from HCC. J Neurooncol 2009; 91:307.
- Shao YY, Lu LC, Cheng AL, Hsu CH. Increasing incidence of brain metastasis in patients with advanced hepatocellular carcinoma in the era of antiangiogenic targeted therapy. Oncologist 2011; 16:82.
- Chen DS, Sung JL, Sheu JC, et al. Serum alpha-fetoprotein in the early stage of human hepatocellular carcinoma. Gastroenterology 1984; 86:1404
- Torzilli G, Minagawa M, Takayama T, et al. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology 1999; 30:889.
- Levy I, Greig PD, Gallinger S, et al. Resection of hepatocellular carcinoma without preoperative tumor biopsy. Ann Surg 2001; 234:206.
- Jia GS, Feng GL, Li JP, et al. Using receiver operating characteristic curves to evaluate the diagnostic value of the combination of multislice spiral CT and alpha-fetoprotein levels for small hepatocellular carcinoma in cirrhotic patients. Hepatobiliary Pancreat Dis Int 2017; 16:303.
- Borel F, Konstantinova P, Jansen PL. Diagnostic and therapeutic potential of miRNA signatures in patients with hepatocellular carcinoma. J Hepatol 2012; 56:1371.
- Zhou J, Yu L, Gao X, et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol 2011; 29:4781.
- Li L, Guo Z, Wang J, et al. Serum miR-18a: a potential marker for hepatitis B virus-related hepatocellular carcinoma screening. Dig Dis Sci 2012; 57:2910.
- Nomura F, Ishijima M, Horikoshi A, et al. Determination of serum des-gamma-carboxy prothrombin levels in patients with small-sized hepatocellular carcinoma: comparison of the conventional enzyme immunoassay and two modified methods. Am J Gastroenterol 1996; 91:1380.
- Marrero JA, Su GL, Wei W, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology 2003; 37:1114.
- Leerapun A, Suravarapu SV, Bida JP, et al. The utility of Lens culinaris agglutinin-reactive alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: evaluation in a United States referral population. Clin Gastroenterol Hepatol 2007; 5:394.
- Toyoda H, Kumada T, Tada T, et al. Clinical utility of highly sensitive Lens culinaris agglutinin-reactive alpha-fetoprotein in hepatocellular carcinoma patients with alpha-fetoprotein <20 ng/mL. Cancer Sci 2011; 102:1025.
- Oda K, Ido A, Tamai T, et al. Highly sensitive lens culinaris agglutinin-reactive α-fetoprotein is useful for early detection of hepatocellular carcinoma in patients with chronic liver disease. Oncol Rep 2011; 26:1227.
- Sterling RK, Jeffers L, Gordon F, et al. Utility of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxy prothrombin, alone or in combination, as biomarkers for hepatocellular carcinoma. Clin Gastroenterol Hepatol 2009; 7:104.
- International Consensus Group for Hepatocellular NeoplasiaThe International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 2009; 49:658.
- Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018; 359:926.
- Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022
- European Association for the Study of the Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943.
- Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474
- Chang TS, Wu YC, Tung SY, et al. Alpha-fetoprotein measurement benefits hepatocellular carcinoma surveillance in patients with cirrhosis. Am J Gastroenterol. 2015;110:836-844
- Llovet JM, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004 Feb. 10(2 Suppl 1):S115-20
- Kim WR. The use of decision analytic models to inform clinical decision making in the management of hepatocellular carcinoma. Clin Liver Dis. 2005 May. 9(2):225-34
- van Leeuwen DJ, Shumate CR. Space-occupying lesions of the liver. van Leeuwen DJ, et al, eds. Imaging in Hepatobiliary and Pancreatic Disease. London: WB Saunders; 2000
- Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006 Mar. 101 (3):513-23
- Lencioni R, Cioni D, Della Pina C, Crocetti L, Bartolozzi C. Imaging diagnosis. Semin Liver Dis. 2005. 25 (2):162-70
- Kulik LM, Mulcahy MF, Omary RA, Salem R. Emerging approaches in hepatocellular carcinoma. J Clin Gastroenterol. 2007 Oct. 41 (9):839-54
- Llovet JM, Fuster J, Bruix J, Barcelona-Clínic Liver Cancer Group. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004 Feb. 10 (2 Suppl 1):S115-20
- Broelsch CE, Frilling A, Malago M. Hepatoma–resection or transplantation. Surg Clin North Am. 2004 Apr. 84(2):495-511, x
- Kemmer N, Neff G, Kaiser T, Zacharias V, Thomas M, Tevar A, et al. An analysis of the UNOS liver transplant registry: high serum alpha-fetoprotein does not justify an increase in MELD points for suspected hepatocellular carcinoma. Liver Transpl. 2006 Oct. 12 (10):1519-22
- Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005 Sep. 11(9):1086-92
- Adapted from Llovet JM, Fuster J, Bruix J, Barcelona-Clinic Liver Cancer Group. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. Feb 2004;10(2 Suppl 1):S115-20.
- Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005 Apr. 41(4):707-16
- Kaido T, Mori A, Ogura Y, Hata K, Yoshizawa A, Iida T, et al. Living donor liver transplantation for recurrent hepatocellular carcinoma after liver resection. Surgery. 2012 Jan. 151 (1):55-60
- A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998; 28:751
- Liu CL, Fan ST, Lo CM, et al. Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol 2001; 19:3725
- Vitale A, Huo TL, Cucchetti A, et al. Survival Benefit of Liver Transplantation Versus Resection for Hepatocellular Carcinoma: Impact of MELD Score. Ann Surg Oncol 2015; 22:1901
- Lobo L, Yakoub D, Picado O, et al. Unresectable Hepatocellular Carcinoma: Radioembolization Versus Chemoembolization: A Systematic Review and Meta-analysis. Cardiovasc Intervent Radiol 2016; 39:1580
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003; 37:429
- Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev 2011; :CD004787.
- Katsanos K, Kitrou P, Spiliopoulos S, et al. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. PLoS One 2017; 12:e0184597.
- Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002; 359:1734.
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002; 35:1164.
- Verslype C, Rosmorduc O, Rougier P, ESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23 Suppl 7:vii41.
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018; 69:182.
- Heimbach J, Kulik LM, Finn R, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2017.
- Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol 2015; 62:1187.
- Lencioni R, Petruzzi P, Crocetti L. Chemoembolization of hepatocellular carcinoma. Semin Intervent Radiol 2013; 30:3.
- Chow PKH, Gandhi M, Tan SB, et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J Clin Oncol 2018; 36:1913.
- Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 2017; 18:1624.
- Bruix J, Cheng AL, Meinhardt G, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol 2017; 67:999.
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10:25.
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378.
- Yoon SM, Ryoo BY, Lee SJ, et al. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol 2018; 4:661
