EPIDEMIOLOGY
In women, breast cancer is the most common type of cancer occurring globally including in Pakistan. Reportedly, Pakistan is the country with highest incidence rate of breast cancer in Asia. Common age bracket of diagnosis with breast cancer in Pakistan is between 31 years to 60 years.(1) In Pakistan at some stage of the life, 1 in 8 women would develop breast cancer.(2)
Data suggests that estimated number of new cases of breast cancer in 2018 in Pakistan were 34,066 per 100,000. Prevalence of breast cancer over 5 years in Pakistan is 73,046 per 100,000 whereas; mortality associated with breast cancer is 17,158 per 100,000. Breast cancer is also ranked top of the list causing deaths due to any type of cancer. Age standardized rate of breast cancer in Pakistan was reported 43.9 per 100,000.(3)
Whereas, globally in 2018 the incidence rate of breast cancer is 2,088,849 per 100,000 with 5-year prevalence rate of 6,875,099 and deaths occurring due to breast cancer was 626,679.(3)
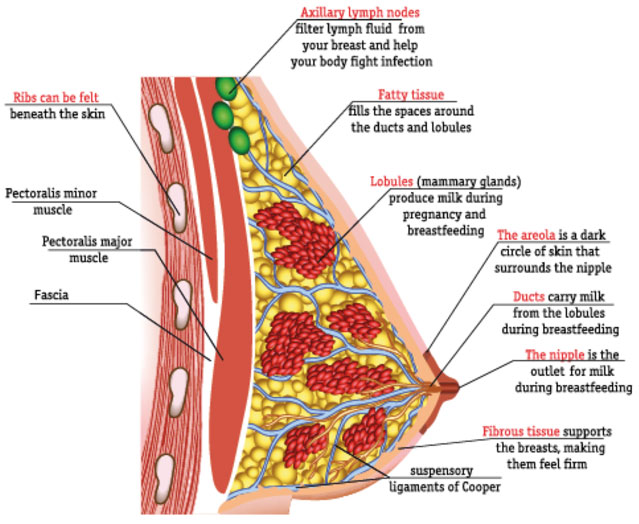
PATHOPHYSIOLOGY(4)
The current understanding of breast cancer etiopathogenesis is that invasive cancers arise through a series of molecular alterations at the cell level. These alterations result in breast epithelial cells with immortal features and uncontrolled growth.
Genomic profiling has demonstrated the presence of discrete breast tumor subtypes with distinct natural histories and clinical behavior. The exact number of disease subtypes and molecular alterations from which these subtypes arise remains to be fully elucidated, but these generally align with the presence or absence of estrogen receptor [ER], progesterone receptor [PR], and human epidermal growth factor receptor 2 (HER2).
This view of breast cancer–not as a set of stochastic molecular events, but as a limited set of separable diseases of distinct molecular and cellular origins–has altered thinking about breast cancer etiology, type-specific risk factors, and prevention and has had a substantial impact on treatment strategies and breast cancer research.
Evidence from The Cancer Genome Atlas Network (TCGA) confirms the following four main breast tumor subtypes, with distinct genetic and epigenetic aberrations.(5) (see the image below):
- Luminal A
- Luminal B
- Basal-like
- HER2-positive
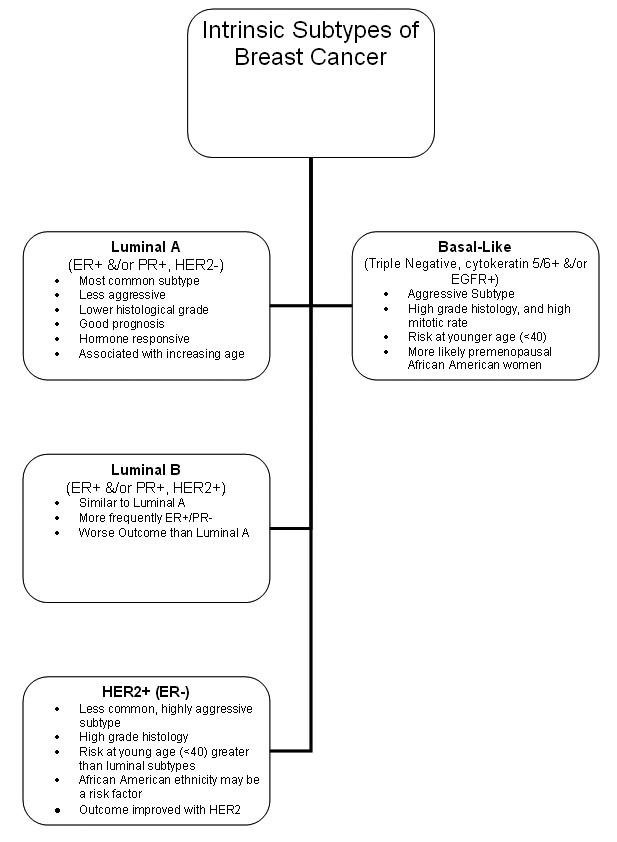
Intrinsic subtypes of breast cancer.
It is noteworthy that the basal-like breast tumor subgroup shares a number of molecular characteristics common to serous ovarian tumors, including the types and frequencies of genomic mutations. These data support the evidence that some breast cancers share etiologic factors with ovarian cancer. Most compelling are the data showing that patients with basal-type breast cancers show treatment responsiveness similar to that of ovarian cancer patients.(6)
The various types of breast cancers are listed below by percentage of cases:
- Infiltrating ductal carcinoma is the most commonly diagnosed breast tumor and has a tendency to metastasize via lymphatics; this lesion accounts for 75% of breast cancers.
- Over the past 25 years, the incidence of lobular carcinoma in situ (LCIS) has doubled, reaching a current level of 2.8 per 100,000 women; the peak incidence is in women aged 40-50 years.
- Infiltrating lobular carcinoma accounts for fewer than 15% of invasive breast cancers.
- Medullary carcinoma accounts for about 5% of cases and generally occurs in younger women.
- Mucinous (colloid) carcinoma is seen in fewer than 5% of invasive breast cancer cases.
- Tubular carcinoma of the breast accounts for 1-2% of all breast cancers.
- Papillary carcinoma is usually seen in women older than 60 years and accounts for approximately 1-2% of all breast cancers.
- Metaplastic breast cancer accounts for fewer than 1% of breast cancer cases, tends to occur in older women (average age of onset in the sixth decade), and has a higher incidence in blacks.
- Mammary Paget disease accounts for 1-4% of all breast cancers and has a peak incidence in the sixth decade of life (mean age, 57 years).
NATURAL HISTORY
The natural history and prognosis for primary breast cancer vary considerably from patient to patient. Some patients present with very indolent disease and either are cured by local therapy or survive for many years even after developing metastases.
The heterogeneity of the natural history of breast cancer complicates patient management. Obviously, patients with virulent, fast-growing tumors might be treated aggressively because of their poor prognosis, whereas other patients with indolent tumors might be spared the morbidity and cost of excessive interventions when their disease is unlikely to compromise survival. A major focus of research in recent years has been the identification of tumor or host factors that would accurately predict patient outcome. The ideal prognostic marker would be one that, if expressed by the tumor, signified early metastases and short survival. Tumors not expressing the marker would be associated with an indolent course, the absence of metastases, and prolonged survival in nearly all patients. Although the ideal prognostic factor does not yet exist, a number of variables have been identified that can help to identify patients according to their relative risk for recurrence. These factors attempt to measure and quantify the degree of tumor differentiation, tumor aggressiveness or metastatic potential, rate of growth or sensitivity/resistance to planned treatment.
SIGN AND SYMPTOMS(4)
History
Many early breast carcinomas are asymptomatic, particularly if they were discovered during a breast-screening program. Larger tumors may present as a painless mass. Pain or discomfort is not usually a symptom of breast cancer; only 5% of patients with a malignant mass present with breast pain.
Often, the purpose of the history is not diagnosis but risk assessment. A family history of breast cancer in a first-degree relative is the most widely recognized breast cancer risk factor.
The US Preventive Services Task Force (USPSTF) has updated its 2005 guidelines on risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women. The current USPSTF recommendations are as follows:(7,8)
- Women who have family members with breast, ovarian, tubal, or peritoneal cancer should be screened to identify a family history that may be associated with an increased risk for mutations in the breast cancer susceptibility genes BRCA1 or BRCA2
- Women who have positive screening results should receive genetic counseling and then BRCA testing if warranted
- Women without a family history associated with an increased risk for mutations should not receive routine genetic counseling or BRCA testing
Physical Examination
If the patient has not noticed a lump, then signs and symptoms indicating the possible presence of breast cancer may include the following:
- Change in breast size or shape
- Skin dimpling or skin changes (e.g. thickening, swelling, or redness)
- Recent nipple inversion or skin change or other nipple abnormalities (e.g. ulceration, retraction, or spontaneous bloody discharge)
- Nipple discharge, particularly if bloodstained
- Axillary lump
To detect subtle changes in breast contour and skin tethering, the examination must include an assessment of the breasts with the patient upright with arms raised. The following findings should raise concern:
- Lump or contour change
- Skin tethering
- Nipple inversion
- Dilated veins
- Ulceration
- Mammary Paget disease
- Edema or peau d’orange
The nature of palpable lumps is often difficult to determine clinically, but the following features should raise concern:
- Hardness
- Irregularity
- Focal nodularity
- Asymmetry with the other breast
- Fixation to skin or muscle (assess fixation to muscle by moving the lump in the line of the pectoral muscle fibers with the patient bracing her arms against her hips)
A complete examination includes assessment of the axillae and supraclavicular fossae, examination of the chest and sites of skeletal pain, and abdominal and neurologic examinations. The clinician should be alert to symptoms of metastatic spread, such as the following:
- Breathing difficulties
- Bone pain
- Symptoms of hypercalcemia
- Abdominal distention
- Jaundice
- Localizing neurologic signs
- Altered cognitive function
- Headache
RATIONALE FOR SCREENING
US Preventive Services Task Force (USPSTF)
In January 2016, the US Preventive Services Task Force (USPSTF) issued its final recommendations on breast cancer screening.(9,10) The guidelines include the following:
- The USPSTF recommends biennial screening mammography for women aged 50 to 74 years
- No requirement for routine screening mammography in women aged 40-49 years (grade C recommendation); the decision to start regular, biennial screening mammography before age 50 years should be an individual one and should take into account patient context, including the patient’s values regarding specific benefits and harms
- Insufficient current evidence to assess the additional benefits and harms of screening mammography in women aged 75 years or older
- Insufficient current evidence to assess the additional benefits and harms of either digital mammography or magnetic resonance imaging (MRI) instead of film mammography as a screening modality for breast cancer
- No requirement for clinicians to teach women how to perform BSE (grade D recommendation); this recommendation is based on studies that found that teaching BSE did not reduce breast cancer mortality but instead resulted in additional imaging procedures and biopsies
- Insufficient current evidence to assess the additional benefits and harms of clinical breast examination (CBE) beyond screening mammography in women aged 40 years or older
American Cancer Society guidelines
The 2015 update of the American Cancer Society guidelines includes the following recommendations:(11)
- Women with an average risk of breast cancer should undergo regular screening mammography starting at age 45 years (strong recommendation).
- Women aged 45 to 54 years should be screened annually (qualified recommendation).
- Women 55 years and older should transition to biennial screening or have the opportunity to continue screening annually (qualified recommendation).
- Women should have the opportunity to begin annual screening between the ages of 40 and 44 years (qualified recommendation).
- Women should continue screening mammography as long as their overall health is good and they have a life expectancy of 10 years or longer (qualified recommendation).
- The ACS does not recommend clinical breast examination for breast cancer screening among average-risk women at any age (qualified recommendation).
American College of Obstetricians and Gynecologists guidelines
The 2017 update of the American College of Obstetricians and Gynecologists guidelines on screening in average-risk women includes the following recommendations for practitioner:(12)
- Use shared decision-making to select screening choices
- Clinical breast examination may be offered every 1-3 years for women aged 29-39 years and annually for women aged ≥ 40 years
- Start offering mammography at age 40 years; initiate after counseling, if patient desires
- Recommend starting mammography screening by no later than age 50 years
- Mammography may be annual or biennial; biennial screening is particularly reasonable after age 55 years
- Continue mammography until age 75 years, then discuss discontinuation, with the woman’s health status and longevity as considerations
National Comprehensive Cancer Network guidelines
The NCCN on screening in average-risk women includes the following recommendations:(13)
- Clinical breast examinations every 1-years from age 25-39, then annually from age 40 on
- Begin annual screening mammography at age 40 years.
- Consider tomosynthesis (three-dimensional mammography)
NCCN guidelines provide four separate sets of recommendations for women at increased risk, on the basis of personal or family history, These include earlier initiation of mammography, in some cases, and consideration or recommendation of annual MRI. Additional considerations include the following:
- An upper age limit for screening is not yet established. Consider severe comorbid conditions limiting life expectancy (e.g. ≤10 years) and whether therapeutic interventions are planned.
- For women with heterogeneous dense breasts and dense breast tissue, recommend counseling on the risks and benefits of supplemental screening.
- Dense breasts limit the sensitivity of mammography and are associated with an increased risk for breast cancer.
- Full-field digital mammography appears to benefit young women and women with dense breasts.
- Multiple studies show that tomosynthesis can decrease callback rates and appears to improve cancer detection. Most studies used double the dose of radiation, but the radiation dose can be minimized by using synthesized 2-D reconstruction.
- Hand-held or automated ultrasound can increase cancer detection, but may increase recall and benign breast biopsies.
- Current evidence does not support the routine use of molecular imaging (e.g. breast-specific gamma imaging, sestamibi scan, or positron emission mammography) as screening procedures, but emerging evidence suggests that these tests may improve detection of early breast cancers in women with mammographically dense breasts. However, the whole-body effective radiation dose with these tests is between 20 –30 times higher than that of mammography.
Current evidence does not support the routine use of thermography or ductal lavage as screening procedures.
DIAGNOSTIC TEST
Positron Emission Tomography
Using a wide range of labeled metabolites (e.g. fluorinated glucose [18 FDG]), positron emission tomography (PET) can detect changes in metabolic activity, vascularization, oxygen consumption, and tumor receptor status.
When PET is combined with computed tomography (CT) to assist in anatomic localization (PET-CT), scans can identify axillary and nonaxillary (e.g. internal mammary or supraclavicular) lymph node metastasis for the purposes of staging locally advanced and inflammatory breast cancer before initiation of neoadjuvant therapy and restaging high-risk patients for local or distant recurrences.
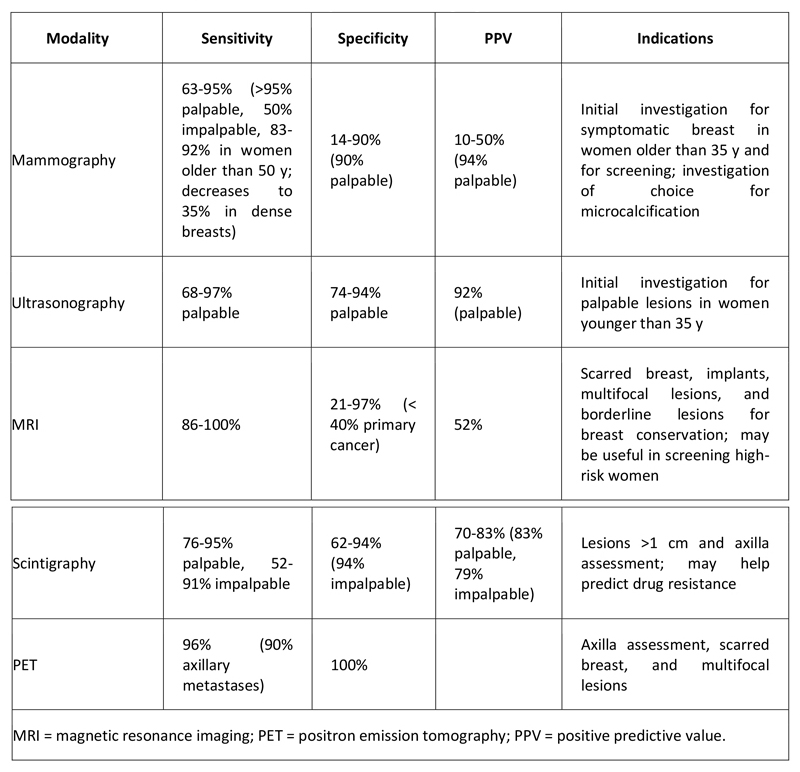
Accuracy of Breast Imaging Modalities
The different techniques used in breast imaging vary with respect to sensitivity, specificity, and positive predictive value (see Table 1 below).
Table 1. Accuracy of Breast Imaging Modalities
Breast Biopsy
Percutaneous vacuum-assisted large-gauge core-needle biopsy (VACNB) with image guidance is the recommended diagnostic approach for newly diagnosed breast tumors. Core biopsies can minimize the need for operative intervention (and subsequent scarring, and provide accurate pathologic diagnosis for appropriate management.
Excisional biopsy, as the initial operative approach, has been shown to increase the rate of positive margins. Open excisional biopsy is reserved for lesions where the diagnosis remains equivocal despite imaging and core biopsy assessment or for benign lesions that the patient chooses to have removed. Because wide clearance of the lesion is usually not the goal in diagnostic biopsies, unnecessary distortion of the breast is thereby avoided. Ongoing audit is essential to help reduce an excessive benign-to-malignant biopsy ratio.
Histology
Breast cancers usually are epithelial tumors of ductal or lobular origin.
All of the following features are important in deciding on a course of treatment for any breast tumor:
- Size
- Status of surgical margin
- Presence or absence of estrogen receptor (ER) and progesterone receptor (PR)
- Nuclear and histologic grade
- Proliferation
- Vascular invasion
- Tumor necrosis
- Quantity of intraductal component
- HER2 status
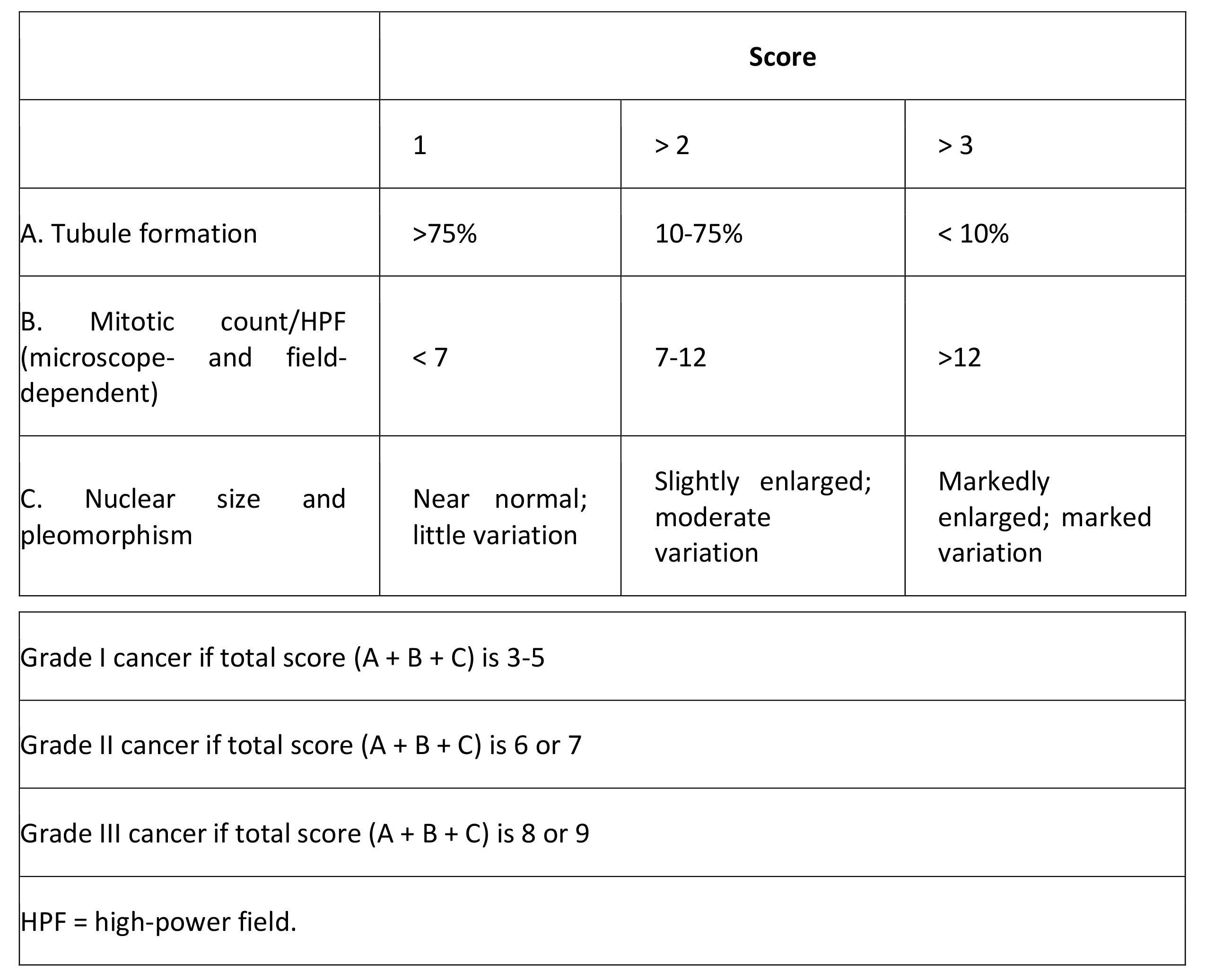
Histologic grade
Histologic grade is the best predictor of disease prognosis in carcinoma in situ, but it is dependent on the grading system used, such as the Van Nuys classification (high-grade, low-grade comedo, low-grade noncomedo). The grading of invasive carcinoma is also important as a prognostic indicator, with higher grades indicating a worse prognosis (see Table 2 below).
Table 2. Grading System in Invasive Breast Cancer (Modified Bloom and Richardson)
Ductal carcinoma in situ: Increased use of screening mammography has resulted in a dramatic increase in the detection of ductal carcinoma in situ (DCIS).DCIS is broadly divided into 2 subtypes: comedo (i.e. cribriform, micropapillary, and solid; and noncomedo. The likelihood of progression or local recurrence, as well as the prognosis, varies in accordance with the DCIS subtype present.
Lobular carcinoma in situ: Lobular carcinoma in situ (LCIS) arises from the terminal duct apparatus and shows a rather diffuse distribution throughout the breast, which explains its presentation as a nonpalpable mass in most cases. Over the past 25 years, the incidence of LCIS has doubled, currently standing at 2.8 per 100,000 women. The peak incidence is in women aged 40-50 years.
Infiltrating ductal carcinoma: Infiltrating ductal carcinoma is the most commonly diagnosed breast tumor (accounting for 75% of breast cancers) and has a tendency to metastasize via lymphatic vessels. This lesion has no specific histologic characteristics other than invasion through the basement membrane. DCIS is a frequently associated finding on pathologic examination.
Infiltrating lobular carcinoma: Infiltrating lobular carcinoma has a much lower incidence than infiltrating ductal carcinoma, accounting for 15-20% of invasive breast cancers. Histologically, it is characterized by the «single-file» arrangement of small tumor cells. Like ductal carcinoma, infiltrating lobular carcinoma typically metastasizes to axillary lymph nodes first. However, it also has a tendency to be multifocal and have discontinuous areas of involvement, making mammographic and even MRI staging imprecise.
Medullary carcinoma: Medullary carcinoma is relatively uncommon (5%) and generally occurs in younger women. Most patients present with a bulky palpable mass and axillary lymphadenopathy. Diagnosis of this type of breast cancer depends on the following histologic triad:
- Sheets of anaplastic tumor cells with scant stroma
- Moderate or marked stromal lymphoid infiltrate
- Histologic circumscription or a pushing border
DCIS may be observed in the surrounding normal tissues. Medullary carcinomas are typically high-grade lesions that are negative for ER, PR, and HER2 and that commonly demonstrate mutation of TP53.
Mucinous carcinoma: Mucinous (colloid) carcinoma is another rare histologic type, seen in fewer than 5% of invasive breast cancer cases. It usually presents during the seventh decade of life as a palpable mass or appears mammographically as a poorly defined tumor with rare calcifications.
Mucin production is the histologic hallmark. There are 2 main types of lesions, A and B, with AB lesions possessing features of both. Type A mucinous carcinoma represents the classic variety, with larger quantities of extracellular mucin, whereas type B is a distinct variant with endocrine differentiation.
DCIS is not a frequent occurrence in this setting, though it may be found. Most cases are ER- and PR-positive, but HER2 overexpression is rare. Additionally, these carcinomas predominantly express glycoproteins MUC2 and MUC6.
Tubular carcinoma: Tubular carcinoma of the breast is an uncommon histologic type, accounting for only 1-2% of all breast cancers. Characteristic features of this type include a single layer of epithelial cells with low-grade nuclei and apical cytoplasmic snoutings arranged in well-formed tubules and glands.
Tubular components make up more than 90% of pure tubular carcinomas and at least 75% of mixed tubular carcinomas. This type of breast cancer has a low incidence of lymph node involvement and a very high overall survival rate. Because of its favorable prognosis, patients are often treated with only breast-conserving surgery and local radiation therapy.
Papillary carcinoma: Papillary carcinoma of the breast encompasses a spectrum of histologic subtypes. There are 2 common types: cystic (noninvasive form) and micropapillary ductal carcinoma (invasive form). This form of breast cancer is usually seen in women older than 60 years and accounts for approximately 1-2% of all breast cancers. Papillary carcinomas are centrally located in the breast and can present as bloody nipple discharge. They are strongly ER- and PR-positive.
Metaplastic breast cancer: Metaplastic breast cancer (MBC) accounts for fewer than 1% of breast cancer cases. It tends to occur in older women (average age of onset in the sixth decade) and has a higher incidence in blacks. It is characterized by a combination of adenocarcinoma plus mesenchymal and epithelial components.
A wide variety of histologic patterns includes the following:
- Spindle-cell carcinoma
- Carcinosarcoma
- Squamous cell carcinoma of ductal origin
- Adenosquamous carcinoma
- Carcinoma with pseudosarcomatous metaplasia
- Matrix-producing carcinoma
This diverse group of malignancies is identified as a single entity on the basis of a similarity in clinical behavior. Compared with infiltrating ductal carcinoma, MBC tumors are larger, faster-growing, commonly node-negative, and typically negative for ER, PR, and HER2.
Mammary Paget disease: Mammary Paget disease is relatively rare, accounting for 1-4% of all breast cancers. The peak incidence is seen in the sixth decade of life. This adenocarcinoma is localized within the epidermis of the nipple-areola complex and is composed of the histologic hallmark Paget cells within the basement membrane. Paget cells are large, pale epithelial cells with hyperchromatic, atypical nuclei, dispersed between the keratinocytes singly or as a cluster of cells.
Lesions are predominantly unilateral, developing insidiously as a scaly, fissured, oozing, or erythematous nipple-areola complex. Retraction or ulceration of the nipple is often noted, along with symptoms of itching, tingling, burning, or pain. In situ or invasive breast cancer is found in approximately 85% of patients with Paget disease. Thus, all diagnosed patients require a careful breast examination and mammographic evaluation, with additional imaging, including breast MRI, if the mammogram is negative.
Breast Cancer Staging
The American Joint Committee on Cancer (AJCC) staging system groups patients into four stages according to the TNM system, which is based on tumor size (T), lymph node status (N), and distant metastasis (M). The forthcoming eighth edition of the AJCC staging system, which will take effect on January 1, 2018, will have changes that include the following:(14)
- Incorporation of immunohistochemically detected tumor markers, to refine prognosis
- Use of genomic assays when available to downstage some estrogen receptor–positive, lymph node–negative tumors
- Removal of lobular carcinoma in situ, because it is not a malignancy but a risk factor
Table 3. TNM Staging System for Breast Cancer
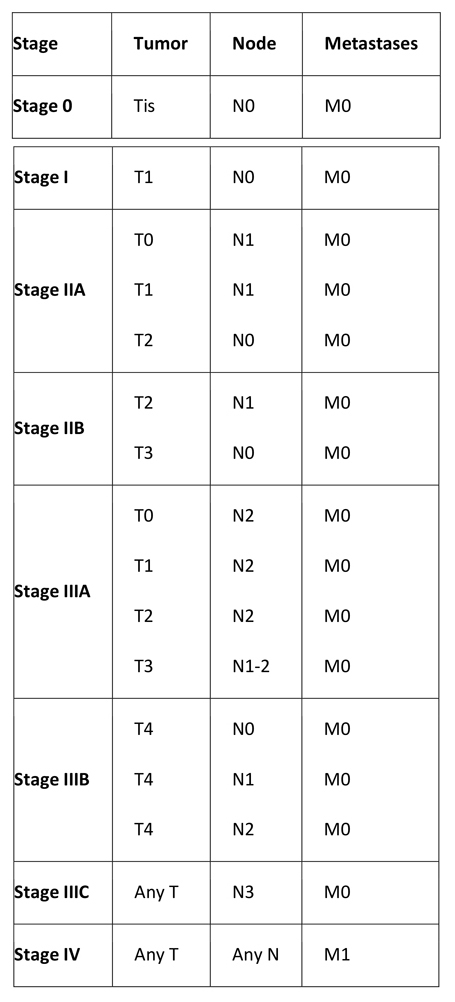
Primary tumor (T)
Tumor size definitions are as follows:
- Tx – Primary tumor cannot be assessed
- T0 – No evidence of primary tumor
- Tis – DCIS
- Tis – LCIS
- Tis – Paget disease of the nipple with no tumor (Paget disease associated with a tumor is classified according to the size of the tumor)
- T1 – Tumor ≤2 cm in greatest diameter
- T1mic – Microinvasion ≤0.1 cm in greatest diameter
- T1a – Tumor >0.1 but not >0.5 cm in greatest diameter
- T1b – Tumor >0.5 but not >1 cm in greatest diameter
- T1c – Tumor >1 cm but not >2 cm in greatest diameter
- T2 – Tumor >2 cm but not >5 cm in greatest diameter
- T3 – Tumor >5 cm in greatest diameter
- T4 – Tumor of any size, with direct extension to (a) the chest wall or (b) skin only, as described below
- T4a – Extension to the chest wall, not including the pectoralis
- T4b – Edema (including peau d’orange) or ulceration of the skin of the breast or satellite skin nodules confined to the same breast
- T4c – Both T4a and T4b
- T4d – Inflammatory disease
Regional lymph nodes (N)
Clinical regional lymph node definitions are as follows:
- Nx – Regional lymph nodes cannot be assessed (e.g. previously removed)
- N0 – No regional lymph node metastasis
- N1 – Metastasis in movable ipsilateral axillary lymph node(s)
- N2 – Metastasis in ipsilateral axillary lymph node(s) fixed or matted, or in clinically apparent ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastasis
- N2a – Metastasis in ipsilateral axillary lymph nodes fixed to one another or to other structures
- N2b – Metastasis only in clinically apparent ipsilateral internal mammary nodes and in the absence of clinically evident axillary lymph nodes
- N3 – Metastasis in ipsilateral infraclavicular or supraclavicular lymph node(s) with or without axillary lymph node involvement, or clinically apparent ipsilateral internal mammary lymph node(s) and in the presence of axillary lymph node
- N3a – Metastasis in ipsilateral infraclavicular lymph node(s)
- N3b – Metastasis in ipsilateral internal mammary lymph node(s) and axillary lymph node(s)
- N3c – Metastasis in ipsilateral supraclavicular lymph node(s)
Distant metastasis
Metastases are defined as follows:
- Mx – Distant metastasis cannot be assessed
- M0 – No distant metastasis
- M1 – Distant metastasis
Lymph node assessment: Evaluation of lymph node involvement by means of sentinel lymph node biopsy or axillary lymph node dissection (ALND) has also been considered necessary for staging and prognosis.
A 2014 update on sentinel lymph node biopsy for patients with early-stage breast cancer by the American Society of Clinical Oncology (ASCO) advises that sentinel lymph node biopsy may be offered to the following patients:(15)
- Women with operable breast cancer and multicentric tumors
- Women with DCIS who will be undergoing mastectomy
- Women who previously underwent breast and/or axillary surgery
- Women who received preoperative/neoadjuvant systemic therapy
According to the ASCO guidelines, sentinel lymph node biopsy should not be performed in patients with any of the following:
- Large or locally advanced invasive breast cancer (tumor size T3/T4)
- Inflammatory breast cancer
- DCIS (when breast-conserving surgery is planned)
- Pregnancy
ASCO recommendations regarding ALND in patients who have undergone sentinel lymph node biopsy are as follows:
- ALND should not be performed in women with no sentinel lymph node (SLN) metastases
- In most cases, ALND should not be performed in women with one to two metastatic SLNs who are planning to undergo breast-conserving surgery with whole-breast radiotherapy
- ALND should be offered to women with SLN metastases who will be undergoing mastectomy
National Comprehensive Cancer Network (NCCN) recommendations differ from those of ASCO in that the NCCN considers that women with clinical stage as high as IIIA T3, N1, M0 may be candidates for SLN biopsy. In addition, the NCCN concluded that there is insufficient evidence to make recommendations for or against SLN biopsy in pregnant patients; the NCCN recommends that decisions regarding use of SLN biopsy in pregnancy be individualized. However, isosulfan blue or methylene blue dye is contraindicated for SLNB in pregnancy; radiolabeled sulfur colloid appears to be safe.(13)
The NCCN breast cancer guidelines state that lymph node dissection is optional in the following cases:(13)
- Strongly favorable tumors
- When no result would affect the choice of adjuvant systemic therapy
- Elderly patients
- Patients with comorbid conditions
Additional Testing
The 2017 NCCN guidelines(13) recommend the following laboratory studies for all asymptomatic women with early-stage breast cancer (stages I–IIB):
- Complete blood count (CBC) with differential
- Comprehensive metabolic panel, with liver function tests (LFTs) and alkaline phosphatase
Additional studies indicated in specific settings include the following:
- Bone scan, in patients with localized bone pain or alkaline phosphatase elevation
- Abdominal ± pelvic diagnostic CT with contrast or MRI with contrast, in patients with elevated alkaline phosphatase, abnormal liver function tests, abdominal symptoms, or abnormal physical examination of the abdomen or pelvis
- Chest diagnostic CT with contrast, in patients with pulmonary symptoms
For women with clinical stage lllA (T3, N1, M0) disease, tests to consider are as follows:
- CBC
- Comprehensive metabolic panel, including LFTs and alkaline phosphatase
- Chest diagnostic CT with contrast
- Abdominal ± pelvic diagnostic CT with contrast or MRI with contrast
- Bone scan or sodium fluoride PET/CT (category 2B)
- FDG PET/CT (optional)
HER2 testing
Although several methods for HER2 testing have been developed, approximately 20% of current HER2 testing may be inaccurate; accordingly, the American Society of Clinical Oncology (ASCO) and CAP have recommended guidelines to ensure the accuracy of HER2 testing. Breast cancer specimens should initially undergo HER2 testing by a validated immunohistochemistry (IHC) assay (e.g. HercepTest; Dako, Glostrup, Denmark) for HER2 protein expression.(16)
The scoring method for HER2 expression is based on the cell membrane staining pattern and is as follows:
- 3+ – Positive for HER2 protein expression; uniform intense membrane staining of more than 30% of invasive tumor cells
- 2+ – Equivocal for HER2 protein expression; complete membrane staining that is either nonuniform or weak in intensity but has circumferential distribution in at least 10% of cells, or uniform intense membrane staining in 30% or less of tumor cells
- 1+ – Weak or incomplete membrane staining in any tumor cells
- 0 – Negative for HER2 protein expression; no staining
Breast cancer specimens with equivocal IHC results should undergo validation with a HER2 gene amplification method, such as fluorescence in situ hybridization (FISH). More centers are relying on FISH alone for determining HER2 status.
In general, FISH testing is thought to be more reliable than IHC, but it is more expensive. Equivocal IHC results can be seen in 15% of invasive breast cancers, whereas equivocal HER2 FISH results are seen in fewer than 3% of invasive breast cancer specimens and those that had previously been considered HER2 positive. Discordant results (IHC 3+/FISH negative or IHC < 3+/FISH positive) have been observed in approximately 4% of specimens. Currently, no data support excluding this group from treatment with trastuzumab.
Newer methodologies for establishing HER2 status, including reverse transcriptase–polymerase chain reaction (RT-PCR) and chromogenic in situ hybridization (CISH), have been developed. The HER2 CISH PharmDX Kit was approved by the FDA in November 2011. The interpretation for HER2 FISH testing (ratio of HER2 to chromosome 17 centromere [HER2/CEP17] and gene copy number) is as follows:
- Positive HER2 amplification – HER2:CEP17 ratio is greater than 2.2 or HER2 gene copy is greater than 6.0
- Equivocal HER2 amplification – HER2:CEP17 ratio of 1.8-2.2 or HER2 gene copy of 4.0-6.0
- Negative HER2 amplification – HER2:CEP17 ratio is less than 1.8 or HER2 gene copy of less than 4.0
Molecular profiling assays
The Onco type Dx assay has been approved by the US Food and Drug Administration (FDA) for women with early-stage ER-positive, node-negative breast cancer treated with tamoxifen, where the recurrence score (RS) correlated with both relapse-free interval and overall survival. This assay is an RT-PCR–based assay of 21 genes (16 cancer genes and 5 reference genes) performed on paraffin-embedded breast tumor tissue.
By using a formula based on the expression of these genes, an RS can be calculated that correlates with the likelihood of distant recurrence at 10 years. Breast tumor RSs and risk levels are as follows:
- < 18, low risk
- 18-30, intermediate risk
- >30, high risk
The MammaPrint assay is a genetic test that measures the activity of 70 genes to determine the 5- to 10-year relapse risk for women diagnosed with early breast cancer. It was approved for use by the FDA in 2007 and is an alternative platform to Oncotype DX. MammaPrint test results are reported as either a low-risk or a high-risk RS:
- A low-risk score means that the cancer has a 10% risk of coming back within 10 years without any additional treatments after surgery
- A high-risk score means that the cancer has a 29% risk of coming back within 10 years without any additional treatments after surgery
THERAPY CONSIDERATIONS
Surgery is considered primary treatment for early-stage breast cancer; many patients are cured with surgery alone. The goals of breast cancer surgery include complete resection of the primary tumor with negative margins to reduce the risk of local recurrences and pathologic staging of the tumor and axillary lymph nodes (ALNs) to provide necessary prognostic information.
Adjuvant treatment of breast cancer is designed to treat micrometastatic disease (i.e. breast cancer cells that have escaped the breast and regional lymph nodes but which have not yet had an established identifiable metastasis). Adjuvant treatment for breast cancer involves radiation therapy and systemic therapy (including a variety of chemotherapeutic, hormonal and biologic agents).
TREATMENT OPTIONS
Treatment of Invasive Breast Cancer
Surgical treatment of invasive breast cancer may consist of lumpectomy or total mastectomy. In breast cancer patients who have clinically negative nodes, surgery typically includes sentinel lymph node (SLN) dissection for staging the axilla.
Lumpectomy margins: The following consensus guideline, released by the Society of Surgical Oncology and the American Society for Radiation Oncology, addresses margins for breast-conserving surgery with whole-breast irradiation (WBI) in stages I and II invasive breast cancer:(17)
- Positive margins are associated with at least a 2-fold increase in ipsilateral breast tumor recurrence (IBTR)
- Negative margins optimize IBTR; this risk is not significantly lowered by wider margin widths
- IBTR rates are reduced with the use of systemic therapy; in patients who do not receive adjuvant systemic therapy, margins wider than no ink on tumor are not needed
- Biologic subtypes do not indicate the need for margins wider than no ink on tumor
- Margin width should not determine the choice of WBI delivery technique, fractionation, and boost dose.
- Wider negative margins than no ink on tumor are not indicated for patients with invasive lobular cancer; classic lobular carcinoma in situ (LCIS) at the margin is not an indication for reexcision; the significance of pleomorphic LCIS at the margin is not clear
- Young age is associated with an increased risk for IBTR after breast-conserving therapy, an increased risk for local relapse on the chest wall after mastectomy, and adverse biologic and pathologic features; an increased margin width does not nullify the increased risk for IBTR in young patients
- An extensive intraductal component (EIC) identifies patients who may have a large residual ductal carcinoma in situ (DCIS) burden after lumpectomy; when margins are negative, there is no evidence of an association between an increased risk for IBTR and EIC
Postlumpectomy radiation therapy: The purpose of radiation therapy after breast-conserving surgery is to eradicate local subclinical residual disease while reducing local recurrence rates by approximately 75%. On the basis of results from several randomized controlled studies, irradiation of the intact breast is considered standard of care, even in the lowest-risk disease with the most favorable prognostic features.
There are 2 general approaches used to deliver radiation therapy: conventional external-beam radiotherapy (EBRT) and partial-breast irradiation (PBI). Whole-breast radiotherapy (WBRT) consists of EBRT delivered to the breast at a dose of 50-55 Gy over 5-6 weeks. This is often followed by a boost dose specifically directed to the area in the breast where the tumor was removed.
Common side effects of radiation therapy include fatigue, breast pain, swelling, and skin desquamation. Late toxicity (lasting ≥6 months after treatment) may include persistent breast edema, pain, fibrosis, and skin hyperpigmentation. Rare side effects include rib fractures, pulmonary fibrosis, cardiac disease (left breast treatment), and secondary malignancies such as radiation-induced sarcoma (0.5%).
PBI is employed in early-stage breast cancer after breast-conserving surgery as a way of delivering larger fraction sizes while maintaining a low risk of late effects. Techniques that can deliver this therapy include interstitial brachytherapy (multiple catheters placed through the breast) and intracavitary brachytherapy (a balloon catheter inserted into the lumpectomy site [i.e. MammoSite]).
Treatment is typically administered twice daily for 5 days. In several nonrandomized studies, these techniques have shown low local recurrence rates comparable to those of EBRT.
Postmastectomy radiation therapy: Clinical practice guidelines developed by the American Society of Clinical Oncology (ASCO), along with several prospective, randomized clinical trials, recommend that postmastectomy radiation therapy be performed according to the following criteria:(6)
- Positive postmastectomy margins
- Primary tumors >5 cm
- Involvement of ≥4 lymph nodes
Patients with more than 4 positive lymph nodes should also undergo prophylactic nodal radiation therapy at doses of 45-50 Gy to the axillary and supraclavicular regions. For patients in whom ALND shows no node involvement, axillary radiation therapy is not recommended.
Systemic Adjuvant Therapy for Breast Cancer
Adjuvant treatment of breast cancer is designed to treat micrometastatic disease (i.e. breast cancer cells that have escaped the breast and regional lymph nodes but which have not yet had an established identifiable metastasis). Treatment is aimed at reducing the risk of future recurrence, thereby reducing breast cancer-related morbidity and mortality. Depending on the model of risk reduction, adjuvant therapy has been estimated to be responsible for 35-72% of the reduction in mortality.
Emerging data suggest that adjuvant therapy with bisphosphonates may prevent disease recurrence and prolong survival. The Early Breast Cancer Trialists’ Collaborative Group found that in postmenopausal women with early breast cancer, adjuvant bisphosphonate therapy produced highly significant reductions in recurrence (rate ratio [RR] 0.86, P=0.002), distant recurrence (RR 0.82, P=0.0003), bone recurrence (RR 0.72, P=0.0002), and breast cancer mortality (RR 0.82, P=0.002). In premenopausal women, bisphosphonate treatment had no apparent effect on any outcome.(19)
Treatment of Carcinoma in Situ
Ductal carcinoma in situ: Currently, the standard treatment of DCIS is surgical resection with or without radiation. Adjuvant radiation and hormonal therapies are often reserved for younger women, patients undergoing lumpectomy, or those with the comedo subtype.
For the majority of patients with DCIS, other approaches might be considered, such as endocrine therapy with tamoxifen/raloxifene or aromatase inhibitors. Women at lowest risk might simply be followed with observation and prevention strategies such as diet, exercise, alcohol moderation, and avoidance of postmenopausal hormone therapy with progesterone-containing regimens.
In DCIS, WBRT is delivered over 5-6 weeks after surgery, reducing the local recurrence rate by approximately 60%. Roughly 50% of local recurrences are invasive breast cancer.
Tamoxifen is the only hormonal therapy currently approved for adjuvant therapy in patients treated with breast-conserving surgery and radiation for DCIS. Adjuvant tamoxifen also reduces the risk of contralateral breast cancer.
Lobular carcinoma in situ: Overall, treatment options for lobular carcinoma in situ (LCIS) include observation and close follow-up care with or without tamoxifen and bilateral mastectomy with or without reconstruction. There is no evidence of therapeutic benefit from local excision, axillary dissection, radiotherapy, or chemotherapy. LCIS in the breast of a woman with ductal or lobular cancer does not require further immediate surgery on the opposite breast. Mirror biopsy of the contralateral breast, once advocated for treatment of LCIS, is now mainly of historic interest.
Treatment of Locally Advanced and Inflammatory Breast Cancer
Originally, the reason for grouping locally advanced breast cancer (LABC) with inflammatory breast cancer (IBC) was the recognition that both diseases had little or no chance of cure from local therapy alone and were therefore considered inoperable. The definition of locally advanced disease has now broadened to include patients who are technically operable but who have large primary tumors (>5 cm).
It is important to recognize, however, that the reasons for using neoadjuvant therapy in women with large primary tumors, in whom the goal is to increase the possibility of breast-conserving surgery, are different from the reasons in women with disease that meets the original criteria of LABC or IBC, for whom the administration of systemic treatment is essential to make definitive local treatment possible with the intent of cure.
In September 2013, the FDA approved pertuzumab for neoadjuvant treatment in combination with trastuzumab and docetaxel for patients with HER2-positive, locally advanced, inflammatory, or early stage breast cancer (either greater than 2 cm in diameter or node positive).
According to evidence-based guidelines from the American Society of Clinical Oncology (ASCO), the HER2 -targeted drugs trastuzumab, pertuzumab, and taxane should be used as first-line therapy for patients with advanced HER2 -positive breast cancer (except in patients with a contraindication to taxane).(20,21) These guidelines provide indications not only for first-line HER2 -targeted therapy but also for second- and third-line treatment.
Overall, the prognosis is better for women with T3N0 (stage IIB) and T3N1 (stage IIIA) breast cancer than it is for those with classically defined LABC (IIIB, IIIC) or IBC (IIIB, T4d). Disease-free survival (DFS) and overall survival are typically better for stage IIB and IIIA patients; however, the likelihood of achieving a pathologic complete response (pCR) from neoadjuvant treatment, a well-recognized surrogate for long-term outcome, is inversely related to tumor size. Thus, the relative proportions of patients in each category are important.
Inflammatory breast cancer: IBC is a clinical diagnosis that implies presentation with the cardinal signs of inflammation (calor [warmth], rubor [redness], tumor [mass]) involving the breast, although the warmth may be subtle and the mass may not be appreciated as something discrete. Indeed, even when a localized mass is apparent in IBC, the true extent of the disease (as shown by performing skin biopsies from the surrounding normal-appearing skin) is usually greater than is apparent on physical examination.
IBC was originally described as having an erysipeloid border. However, only a minority of cases have this component of a raised edge.
These tumors are more likely to stain negatively by IHC for ER and PR and somewhat more likely to be positive for HER2 overexpression. In addition, both angiogenesis and lymphangiogenesis appear to be increased by microvessel density or RNA-based gene expression arrays.
Locally advanced breast cancer: LABC encompasses both relatively indolent neglected tumors and those that have grown rapidly as a result of their inherent biology. In most case series, LABC has a better long-term outcome than IBC does, even when only inoperable cases are considered.
Evaluation of lymph nodes and response: Patients with LABC or IBC with clinically positive nodes should undergo a core biopsy before initiating chemotherapy. Those with clinically negative nodes may undergo sentinel lymph node biopsy before they start treatment, or else sentinel node determination may be delayed until after treatment is completed.
Theoretically, it should be preferable to perform sentinel node sampling up front, because chemotherapy might eradicate preexistent disease in the sentinel lymph node and result in a false-negative result, or altered lymphatic drainage in large tumors might affect accuracy of the procedure.
In general, the best single test for evaluating the status of measurable tumor is ultrasonography (preferably done by the same operator). The mass often appears larger on physical examination than on ultrasonography, which can more effectively discriminate hypoechoic masses from surrounding stroma or hematoma. In IBC, magnetic resonance imaging (MRI) may be an important adjunct to response assessment. The role of positron emission tomography (PET) in routine assessment of response must be determined on a case-by-case basis.
No current imaging technique appears to be highly accurate for the prediction of pCR. Thus, the purposes of regular size assessment are as follows:
- To exclude continuation of therapy in a patient with a growing tumor (seen in < 5% with the initial treatment)
- To suggest when maximal response of grossly evident disease has been achieved (this may be the optimal time to proceed to resection
Systemic Treatment of Metastatic Breast Cancer
Marked advances are being made in the treatment of early-stage breast cancer, but many women still develop recurrence and metastasis. In addition, 5-10% of breast cancer patients have metastatic disease at presentation. Although treatments for metastatic breast cancer continue to improve, there remains no cure once distant metastases develop.
Furthermore, although occasional patients with metastatic breast cancer benefit from surgical resection for an isolated recurrence and many require radiation therapy for palliation at a specific site (or definitive treatment of brain metastasis), in general, recurrent or metastatic breast cancer must be approached systemically so that the therapeutic effect reaches all sites of disease. There are two main interventions: hormone therapy and chemotherapy.
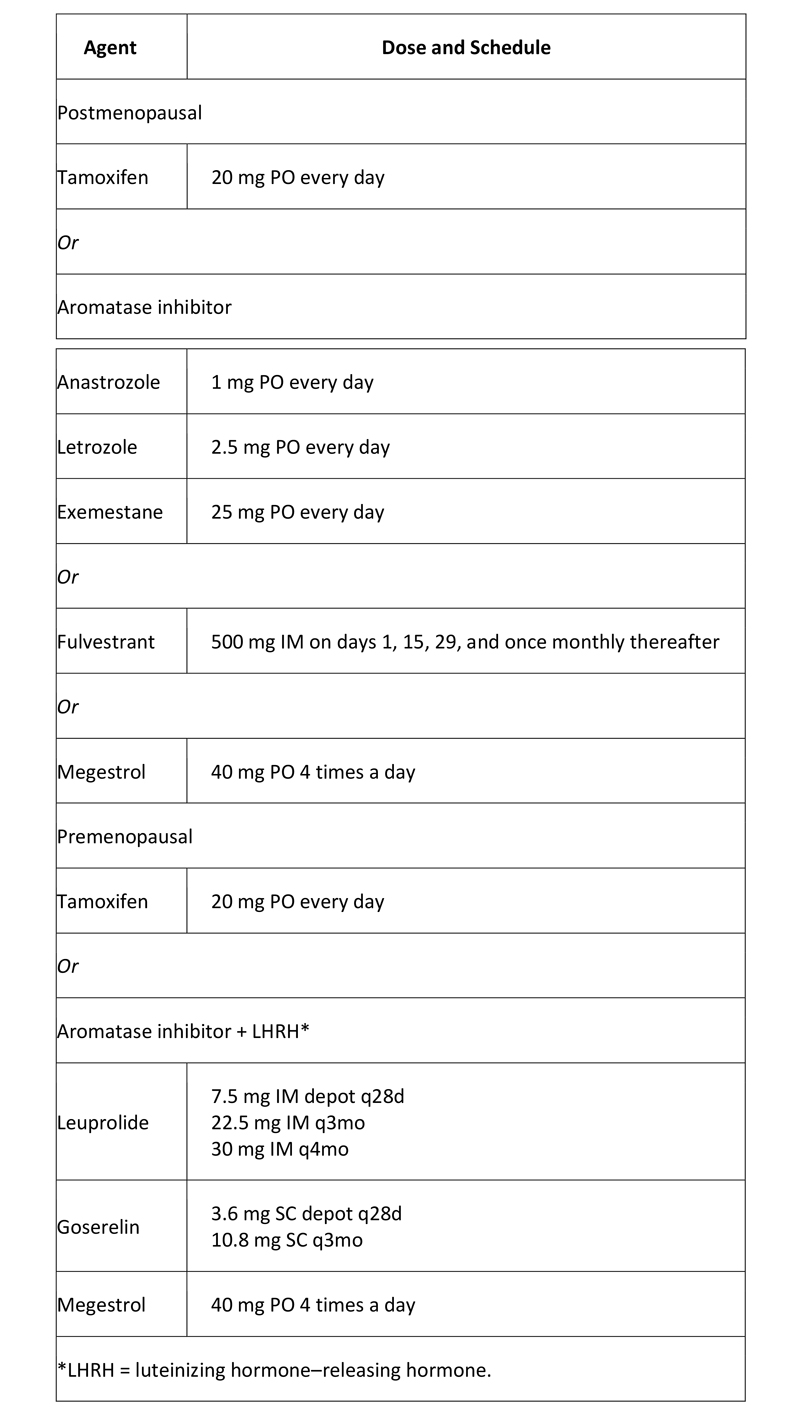
Hormone therapy: For patients who have hormone receptor (ER and/or PR)–positive disease without a life-threatening component (e.g. massive liver metastases) or systemic symptoms requiring immediate palliation for comfort, in general, hormone manipulation is the initial treatment of choice. Response rates are higher with chemotherapy, but so is the incidence of potentially dangerous toxicity, and there is no evidence that patients live longer as a result of receiving initial chemotherapy.
For ER–positive metastatic breast cancer, the American Society of Clinical Oncology (ASCO) recommends using endocrine therapy rather than chemotherapy as first-line treatment, except in patients with immediately life-threatening disease or if there are concerns about endocrine resistance. The recommendation is part of an ASCO clinical practice guideline on the use of chemotherapy and targeted therapy for women with human epidermal growth factor 2 (HER2)-negative (or unknown) advanced breast cancer, with recommendations based on a systematic review of 79 studies.(22)
Table 4. Hormone Agents Used in Breast Cancer
Chemotherapy: Cytotoxic chemotherapy for metastatic breast cancer initially consisted of single-agent regimens. Combination therapy is currently considered up front, depending on the patient’s performance status, because of higher response rates. However, in the setting of advanced disease, the goal in determining a treatment regimen should be to prolong survival while maintaining a good quality of life.
When the patient has life-threatening disease and/or severe symptoms that require quick relief, combinations of cytotoxic agents may be preferable because of their high response rate and early onset of clinical benefit.
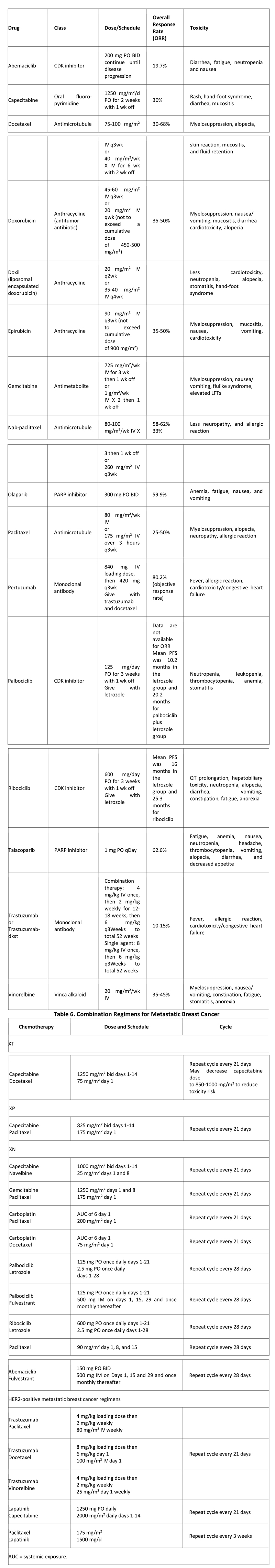
A second situation, which is becoming increasingly common, is when a cytotoxic chemotherapeutic agent is combined with a targeted agent other than hormone therapy. These targeted agents often have very low response rates when given as monotherapy, but they provide added benefit when given in combination with cytotoxic chemotherapy. A list of targeted chemotherapeutic agents is provided in Table 5, below, followed by Table 6, showing combination regimens for breast cancer.
Table 5. Targeted Chemotherapy for Metastatic Breast Cancer
The initial choice of chemotherapy is highly influenced by the patient’s personal history of previous drug exposure. For example, if doxorubicin was a component of previous adjuvant therapy, the tumor cells have a higher risk of developing resistance, and there is a relationship between cumulative lifetime total dose of doxorubicin and the risk of potentially fatal cardiomyopathy.
It is important to realize that if 1 year or more has elapsed since completion of adjuvant therapy, a patient’s tumor is likely to respond to a previously given drug or combination as though that drug or combination had never been given. Most patients have been exposed to both an anthracycline (i.e. doxorubicin) and a taxane (docetaxel or paclitaxel) in the adjuvant setting.
Treatment of breast cancer with a taxane in the metastatic setting after treatment in the adjuvant setting may be difficult because of residual toxicity. Although taxanes are not cardiotoxic, they can produce lingering neuropathy (especially paclitaxel) or problems with edema (docetaxel especially), which makes further administration problematic. Substitution of one taxane for another is possible, depending on the nature of the chronic toxicity.
If the tumor has recurred quickly after administration of adjuvant chemotherapy containing a taxane, then changing the schedule of administration can be effective. At least one third of breast cancer patients with taxane resistance due to administration of every-3-week paclitaxel show a response when the same drug is administered on a weekly schedule at a lower dose.
In addition to taxanes and anthracyclines, a variety of other chemotherapeutic agents can be used as single agents or in combination with taxanes. Capecitabine is an oral agent that essentially represents a sustained-release formulation of the older antimetabolite fluorouracil (5-FU) and provides the convenience of self-administration.
Drugs such as capecitabine have very little associated myelosuppression, and they are often chosen when the patient’s bone marrow has been damaged by previous therapy or when there is a desire to coadminister a myelosuppressive agent for more rapid effect. As a single agent, capecitabine has an ORR of 25-30%, with minimal toxicity. When combined with a taxane, an ORR of 40-50% has been observed, along with a median overall survival benefit of 3-15 months.
Another antimetabolite, gemcitabine, is typically given in combination with paclitaxel, based on results from a phase III trial comparing paclitaxel with the combination regimen in locally advanced breast cancer (LABC) and metastatic breast cancer.
Vinorelbine is a vinca alkaloid that targets tubulin in the mitotic spindle and is administered intravenously, usually on a weekly basis. Vinorelbine is often used as a single agent following treatment with a taxane or anthracycline, yielding an ORR of 25%. However, when used as a first- or second-line agent, vinorelbine can have ORRs of up to 40%.
Palbociclib is an inhibitor of cyclin-dependent kinases (CDKs) 4 and 6 used for first-line treatment for ER-positive, HER2-negative metastatic breast cancer in postmenopausal women, in combination with the aromatase inhibitor letrozole.]
The CDK 4,6 inhibitor ribociclib was approved by the FDA in March 2017 for postmenopausal HR+/HER- advanced or metastatic breast cancer in combination with letrozole.
PARP Inhibitors: Olaparib inhibits poly (ADP-ribose) polymerase (PARP) enzymes. In January 2018, the FDA expanded approval of olaparib to include treatment of BRCA-mutated, HER2-negative metastatic breast cancer in patients who have been previously treated with chemotherapy. Olaparib (which had previously been approved for treatment of BRCA-mutated ovarian cancer) is the first PARP inhibitor approved to treat breast cancer, and the first drug of any kind approved to treat certain patients with BRCA-mutated metastatic breast cancer.
In October 2018, talazoparib, another PARP inhibitor, was approved for patients with deleterious or suspected deleterious germline BRCA-mutated HER2-negative locally advanced or metastatic breast cancer.
Treatment of triple-negative metastatic breast cancer: Unresectable metastatic triple-negative breast cancer (i.e. estrogen receptor–, progesterone receptor–, and HER2 receptor–negative) is aggressive and carries a poor prognosis. However, combination therapy with the programmed cell death ligand–1 (PDL1) inhibitor atezolizumab plus nanoparticle albumin-bound (nab)–paclitaxel has been shown to prolong PFS in these patients.
Antiangiogenic therapy in metastatic breast cancer: Angiogenesis is recognized as a key process in the progression and metastasis of breast cancer. Bevacizumab is a humanized mAb directed against vascular endothelial growth factor (VEGF), which exerts an independent effect on the process of new blood vessel formation in tumors (angiogenesis). Bevacizumab was approved by the FDA as a first-line therapy for HER2-negative metastatic breast cancer patients.
However, on November 18, 2011, the FDA officially rescinded its approval of bevacizumab because the drug had not been shown to be safe and effective for this use.
Surgical Treatment of Metastatic Breast Cancer
As modern systemic chemotherapy has become more effective, some patients with intact primary tumors and metastasis can have long-term stable distant disease or even no evidence of residual metastatic disease after treatment. There is increasing interest in the role of surgical intervention for the intact primary tumor of these metastatic breast cancer patients. Several single-institution cohort and retrospective studies have concluded that surgical resection of the intact primary tumor may provide a survival advantage.
It is still unknown whether a selection bias affects the findings of a survival advantage in favor of surgery. However, the dogmatic belief that one should never operate in the setting of metastatic disease has certainly been dispelled in favor of critical evaluation of whether surgically achieved local control can lead to improved survival as a part of multimodal treatment.
Prophylactic Mastectomy
Prophylactic mastectomy is an option for women found to be at extremely elevated risk for breast cancer. Either total mastectomy or subcutaneous (nipple-sparing) mastectomy may be performed.
Genetic factors that place a woman at very high risk of developing breast cancer include the following:
- Strong family history of breast and/or ovarian cancer
- Pathogenic mutation in BRCA1 or BRCA2
- High-penetrance mutation in another gene associated with breast cancer risk (e.g. TP53, PTEN)
The National Comprehensive Cancer Network (NCCN) recommends that in general, the only women who should consider risk-reduction mastectomy are those with a genetic mutation that confers a high risk for breast cancer, a compelling family history, or possibly a personal history of receiving thoracic radiation therapy before 30 years of age. The NCCN notes that while risk-reduction mastectomy had previously been considered for lobular carcinoma in situ (LCIS), risk-reduction therapy is currently the preferred approach for LCIS.
Woman who are considering prophylactic mastectomy should meet with a range of specialists to discuss the risks and benefits of surgery, including its potential psychosocial effects, as well as the nonsurgical options for reducing risk of breast cancer.These may include a breast health specialist, medical social worker, or cancer clinical psychologist or psychiatrist. Early consultation with a reconstructive surgeon is recommended for those considering either immediate or delayed breast reconstruction.
Contralateral prophylactic mastectomy: A consensus statement from the American Society of Breast Surgeons (ASBrS) recommends that women with unilateral breast cancer who are at average risk should be discouraged from undergoing a contralateral prophylactic mastectomy (CPM), because most of those women, with the possible exception of BRCA carriers, will not obtain a survival benefit, and CPM doubles the risk of surgical complications.
However, the ASBrS advises that the final decision whether or not to proceed with contralateral prophylactic mastectomy is a result of the balance between benefits and risks of CPM and patient preference.
The ASBrS concluded that CPM should be considered for patients with any of the following significant risk factors for contralateral breast cancer:
- BRCA1/2 mutations
- Strong family history (in patient who have not undergone genetic testing)
- Mantle chest radiation before age 30 years
The ASMBrS suggests that CPM can be considered for women with factors that place them at lower risk. These include women who are carriers of a non-BRCA gene (e.g. CHEK-2, PALB2, p53, CDH1) and those with a strong family history of breast cancer but who are themselves BRCA negative and have no family member with known BRCA.
Other reasons for considering CPM, according to the ASMBrS, include the following:
- To limit contralateral breast surveillance (dense breasts, failed surveillance, recall fatigue)
- To improve reconstructed breast symmetry
- To manage risk aversion
- To manage extreme anxiety (although that may be better managed through psychological support strategies)
The ASMBrS recommends discouraging CPM not only in average-risk women with unilateral breast cancer, but in those with any of the following:
- Advanced index cancer (e.g. inflammatory breast cancer, T4 or N3 disease, stage IV disease)
- High risk for surgical complications (e.g. due to comorbidities such obesity, smoking, diabetes)
- Negative BRCA test results, despite a family history of BRCA carriage
Finally, the ASMBrS recommends against CPM in men with breast cancer, even if they are BRCA carriers.The initial choice of chemotherapy is highly influenced by the patient’s personal history of previous drug exposure. For example, if doxorubicin was a component of previous adjuvant therapy, the tumor cells have a higher risk of developing resistance, and there is a relationship between cumulative lifetime total dose of doxorubicin and the risk of potentially fatal cardiomyopathy.
It is important to realize that if 1 year or more has elapsed since completion of adjuvant therapy, a patient’s tumor is likely to respond to a previously given drug or combination as though that drug or combination had never been given. Most patients have been exposed to both an anthracycline (i.e. doxorubicin) and a taxane (docetaxel or paclitaxel) in the adjuvant setting.
Treatment of breast cancer with a taxane in the metastatic setting after treatment in the adjuvant setting may be difficult because of residual toxicity. Although taxanes are not cardiotoxic, they can produce lingering neuropathy (especially paclitaxel) or problems with edema (docetaxel especially), which makes further administration problematic. Substitution of one taxane for another is possible, depending on the nature of the chronic toxicity.
If the tumor has recurred quickly after administration of adjuvant chemotherapy containing a taxane, then changing the schedule of administration can be effective. At least one third of breast cancer patients with taxane resistance due to administration of every-3-week paclitaxel show a response when the same drug is administered on a weekly schedule at a lower dose.
In addition to taxanes and anthracyclines, a variety of other chemotherapeutic agents can be used as single agents or in combination with taxanes. Capecitabine is an oral agent that essentially represents a sustained-release formulation of the older antimetabolite fluorouracil (5-FU) and provides the convenience of self-administration.
Drugs such as capecitabine have very little associated myelosuppression, and they are often chosen when the patient’s bone marrow has been damaged by previous therapy or when there is a desire to coadminister a myelosuppressive agent for more rapid effect. As a single agent, capecitabine has an ORR of 25-30%, with minimal toxicity. When combined with a taxane, an ORR of 40-50% has been observed, along with a median overall survival benefit of 3-15 months.
Another antimetabolite, gemcitabine, is typically given in combination with paclitaxel, based on results from a phase III trial comparing paclitaxel with the combination regimen in locally advanced breast cancer (LABC) and metastatic breast cancer.
Vinorelbine is a vinca alkaloid that targets tubulin in the mitotic spindle and is administered intravenously, usually on a weekly basis. Vinorelbine is often used as a single agent following treatment with a taxane or anthracycline, yielding an ORR of 25%. However, when used as a first- or second-line agent, vinorelbine can have ORRs of up to 40%.
Palbociclib is an inhibitor of cyclin-dependent kinases (CDKs) 4 and 6 used for first-line treatment for ER-positive, HER2-negative metastatic breast cancer in postmenopausal women, in combination with the aromatase inhibitor letrozole.]
The CDK 4,6 inhibitor ribociclib was approved by the FDA in March 2017 for postmenopausal HR+/HER- advanced or metastatic breast cancer in combination with letrozole.
PARP Inhibitors: Olaparib inhibits poly (ADP-ribose) polymerase (PARP) enzymes. In January 2018, the FDA expanded approval of olaparib to include treatment of BRCA-mutated, HER2-negative metastatic breast cancer in patients who have been previously treated with chemotherapy. Olaparib (which had previously been approved for treatment of BRCA-mutated ovarian cancer) is the first PARP inhibitor approved to treat breast cancer, and the first drug of any kind approved to treat certain patients with BRCA-mutated metastatic breast cancer.
In October 2018, talazoparib, another PARP inhibitor, was approved for patients with deleterious or suspected deleterious germline BRCA-mutated HER2-negative locally advanced or metastatic breast cancer.
Treatment of triple-negative metastatic breast cancer: Unresectable metastatic triple-negative breast cancer (i.e. estrogen receptor–, progesterone receptor–, and HER2 receptor–negative) is aggressive and carries a poor prognosis. However, combination therapy with the programmed cell death ligand–1 (PDL1) inhibitor atezolizumab plus nanoparticle albumin-bound (nab)–paclitaxel has been shown to prolong PFS in these patients.
Antiangiogenic therapy in metastatic breast cancer: Angiogenesis is recognized as a key process in the progression and metastasis of breast cancer. Bevacizumab is a humanized mAb directed against vascular endothelial growth factor (VEGF), which exerts an independent effect on the process of new blood vessel formation in tumors (angiogenesis). Bevacizumab was approved by the FDA as a first-line therapy for HER2-negative metastatic breast cancer patients.
However, on November 18, 2011, the FDA officially rescinded its approval of bevacizumab because the drug had not been shown to be safe and effective for this use.
Surgical Treatment of Metastatic Breast Cancer
As modern systemic chemotherapy has become more effective, some patients with intact primary tumors and metastasis can have long-term stable distant disease or even no evidence of residual metastatic disease after treatment. There is increasing interest in the role of surgical intervention for the intact primary tumor of these metastatic breast cancer patients. Several single-institution cohort and retrospective studies have concluded that surgical resection of the intact primary tumor may provide a survival advantage.
It is still unknown whether a selection bias affects the findings of a survival advantage in favor of surgery. However, the dogmatic belief that one should never operate in the setting of metastatic disease has certainly been dispelled in favor of critical evaluation of whether surgically achieved local control can lead to improved survival as a part of multimodal treatment.
Prophylactic Mastectomy
Prophylactic mastectomy is an option for women found to be at extremely elevated risk for breast cancer. Either total mastectomy or subcutaneous (nipple-sparing) mastectomy may be performed.
Genetic factors that place a woman at very high risk of developing breast cancer include the following:
- Strong family history of breast and/or ovarian cancer
- Pathogenic mutation in BRCA1 or BRCA2
- High-penetrance mutation in another gene associated with breast cancer risk (e.g. TP53, PTEN)
The National Comprehensive Cancer Network (NCCN) recommends that in general, the only women who should consider risk-reduction mastectomy are those with a genetic mutation that confers a high risk for breast cancer, a compelling family history, or possibly a personal history of receiving thoracic radiation therapy before 30 years of age. The NCCN notes that while risk-reduction mastectomy had previously been considered for lobular carcinoma in situ (LCIS), risk-reduction therapy is currently the preferred approach for LCIS.
Woman who are considering prophylactic mastectomy should meet with a range of specialists to discuss the risks and benefits of surgery, including its potential psychosocial effects, as well as the nonsurgical options for reducing risk of breast cancer.These may include a breast health specialist, medical social worker, or cancer clinical psychologist or psychiatrist. Early consultation with a reconstructive surgeon is recommended for those considering either immediate or delayed breast reconstruction.
Contralateral prophylactic mastectomy: A consensus statement from the American Society of Breast Surgeons (ASBrS) recommends that women with unilateral breast cancer who are at average risk should be discouraged from undergoing a contralateral prophylactic mastectomy (CPM), because most of those women, with the possible exception of BRCA carriers, will not obtain a survival benefit, and CPM doubles the risk of surgical complications.
However, the ASBrS advises that the final decision whether or not to proceed with contralateral prophylactic mastectomy is a result of the balance between benefits and risks of CPM and patient preference.
The ASBrS concluded that CPM should be considered for patients with any of the following significant risk factors for contralateral breast cancer:
- BRCA1/2 mutations
- Strong family history (in patient who have not undergone genetic testing)
- Mantle chest radiation before age 30 years
The ASMBrS suggests that CPM can be considered for women with factors that place them at lower risk. These include women who are carriers of a non-BRCA gene (e.g. CHEK-2, PALB2, p53, CDH1) and those with a strong family history of breast cancer but who are themselves BRCA negative and have no family member with known BRCA.
Other reasons for considering CPM, according to the ASMBrS, include the following:
- To limit contralateral breast surveillance (dense breasts, failed surveillance, recall fatigue)
- To improve reconstructed breast symmetry
- To manage risk aversion
- To manage extreme anxiety (although that may be better managed through psychological support strategies)
The ASMBrS recommends discouraging CPM not only in average-risk women with unilateral breast cancer, but in those with any of the following:
- Advanced index cancer (e.g. inflammatory breast cancer, T4 or N3 disease, stage IV disease)
- High risk for surgical complications (e.g. due to comorbidities such obesity, smoking, diabetes)
- Negative BRCA test results, despite a family history of BRCA carriage
Finally, the ASMBrS recommends against CPM in men with breast cancer, even if they are BRCA carriers.
GOALS OF THERAPY
The key goal in the treatment of breast cancer is to prolong survival, with an emphasis on restricting treatment-related toxicity as much as possible.
GUIDELINES
To view, “NCCN guidelines on Breast cancer”, please click on the link below.
http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site
To view, “ASCO guidelines on Breast cancer”, please click on the link below.
http://ascopubs.org/jco/site/misc/specialarticles.xhtml
To view, “ESMO Clinical guidelines on breast cancer”, please click on the below link.
http://www.esmo.org/Guidelines/Breast-Cancer/Primary-Breast-Cancer
To view, “U.S preventive services task force – Breast cancer screening guidelines”, please click on below link:
http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm
To view, American cancer society guidelines on Breast cancer”, please click on below link:
https://www.cancer.org/cancer/breast-cancer.html
To view, “American College of Obstetricians and Gynecologists (ACOG) Breast cancer risk assessment and screening in average risk women”, please click on below link:
https://www.acog.org/-/media/Practice-Bulletins/Committee-on-Practice-Bulletins—-Gynecology/Public/pb179.pdf?dmc=1&ts=20180204T1824108792
CONSULTATION AND LONG TERM MONITORING
There is no consensus among oncologists as to appropriate and optimal follow-up for long-term breast cancer survivors. The majority of relapses, both local and distant, occur within the first 3 years, especially in higher-risk and ER-negative patients. The 2007 ASCO guidelines do not support the use of tumor biomarkers, including CEA, CA15.3, and CA27.29, for monitoring patients for recurrence after primary breast cancer therapy. ASCO and NCCN have both provided recommendations for surveillance in the adjuvant setting (see Table 7 below).
Table 7. Follow-up Recommendations for Breast Cancer Survivors
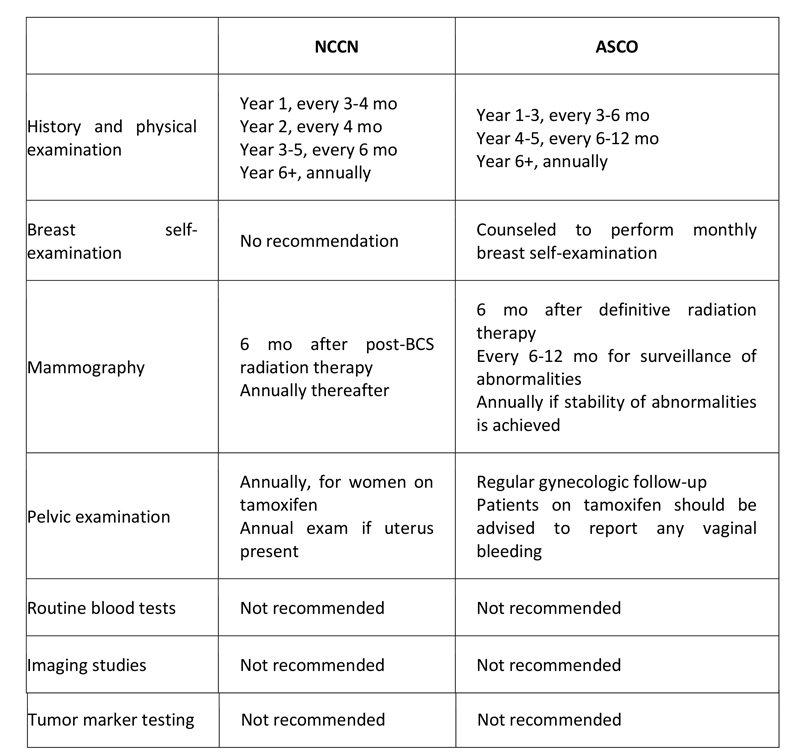
Table 8. ASCO guidelines for monitoring bone density
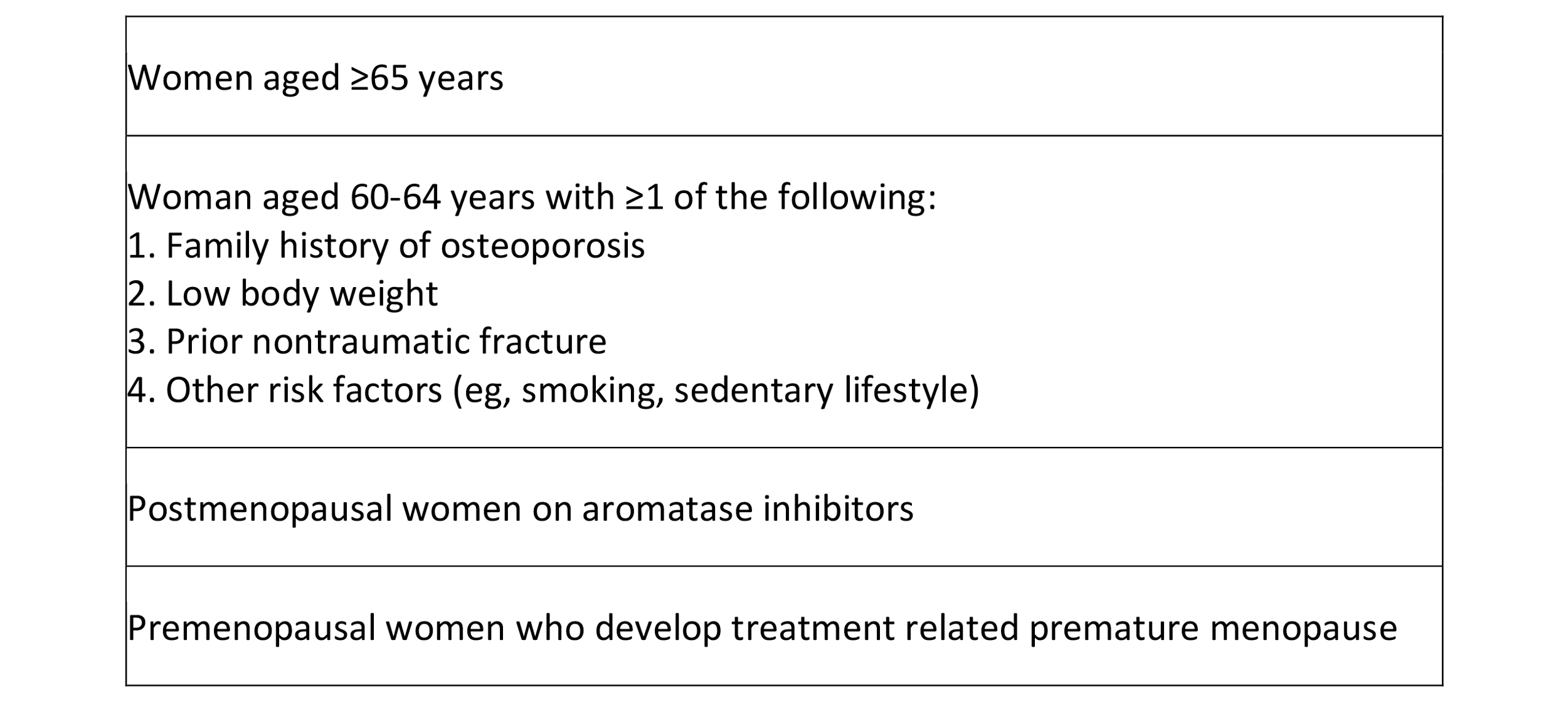
Postoperative imaging
Women who have had surgery for breast cancer may still require breast cancer screening with mammography. If a woman had a total mastectomy, then the other breast requires yearly follow-up, because there is still a higher risk that cancer will develop in the remaining breast. If the woman had a subcutaneous mastectomy, partial mastectomy, or lumpectomy, then that breast itself requires follow-up mammography.
The first mammogram is best performed 6 months postoperatively to provide a baseline for the new postoperative and postirradiation changes. Thereafter, mammography may be performed every 6-12 months for screening and follow-up.
Monitoring of metastatic disease
Recommendations for monitoring disease response in the metastatic setting vary. In general, monthly evaluations consisting of a history and physical examination to evaluate progression of disease and toxicities are reasonable.
Measurement of tumor markers, such as CEA, CA15.3, and CA27.29, can be used in conjunction with diagnostic imaging, history, and physical examination for monitoring patients on active therapy. CA15.3 and CA27.29 levels correlate with the course of disease in 60-70% of patients, whereas CEA levels correlate in 40% of patients.
Standardized guidelines for imaging are not yet established; the choice and timing of imaging procedures should be tailored to each patient’s specific needs. In general, computed tomography (CT) of the chest, abdomen, and pelvis; MRI; bone scanning; or PET-CT is performed when symptoms change or tumor markers rise.
PRECAUTIONS
It is not sure that these measures can prevent breast cancer but however they might help to lower risk of developing breast cancer. Following are some measures that might help you to lower the risk of developing breast cancer.
- Optimum body weight: Weight gain and overweight are both risk factors for developing breast cancer. It is advised to maintain optimum body weight through proper diet and exercise to stay healthy.
- Physically active: Less physical activities are associated with risk for developing breast cancer. However, many studies supported the fact that moderate to intense physical activities can reduce the risk of developing breast cancer. It may include brisk walk, yoga, weight lifting etc.
- Avoid alcohol: Alcohol consumption is directly linked with risk of developing breast cancer and other cancers also. It is advised to limit or avoid alcohol use.
- Other factors: Breast feeding for several months and avoid use of hormone therapy after menopause may also help you to avoid risk of developing breast cancer.
REFERENCES
- Soomro, R., et al., Age and stage of breast cancer in Pakistan: An experience at a tertiary care center. JPMA. The Journal of the Pakistan Medical Association, 2018. 68(11): p. 1682-1685.
- Gulzar, F., Identifying the Barriers of Delayed Presentation in Pakistani Breast Cancer Patients Undergoing Care at Tertiary Hospital: Access to care. 2018, American Society of Clinical Oncology.
- Bray, F., et al., Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 2018. 68(6): p. 394-424.
- Pavani Chalasani. Breast cancer [Internet]. Medscape. Available from: https://emedicine.medscape.com/article/1947145-overview#a5 [accessed on 2019 Jan 09]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012 Oct 4. 490(7418):61-70.
- Recht A, Edge SB, Solin LJ, Robinson DS, Estabrook A, Fine RE, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001 Mar 1. 19(5):1539-69.
- Brooks M. BRCA Testing Update: ‘Do This, Don’t Do That.’. Medscape Medical News. Available at http://www.medscape.com/viewarticle/818267. December 23, 2013; Accessed: February 2, 2018
- Moyer VA. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer in Women: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013 Dec 24
- Siu AL, U.S. Preventive Services Task Force. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016 Feb 16. 164 (4):279-96
- S. Preventive Services Task Force. Screening for Breast Cancer. Available at http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1. 2016; Accessed: November 5, 2018
- Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update From the American Cancer Society. JAMA. 2015 Oct 20. 314 (15):1599-614
- Committee on Practice Bulletins—Gynecology. Practice Bulletin Number 179: Breast Cancer Risk Assessment and Screening in Average-Risk Women. Obstet Gynecol. 2017 Jul. 130 (1):e1-e16
- NCCN Clinical Practice Guidelines in Oncology. Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network. Available at https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf. Version 3.2018 — October 4, 2018
- Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017 Mar 14
- Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014 May 1. 32(13):1365-83
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007 Jan 1. 25(1):118-45
- Chustecka Z. New guideline on lumpectomy margins should reduce re-excision. Medscape Medical News. February 11, 2014.
- Moran MS, Schnitt SJ, Giuliano AE, Harris JR, Khan SA, Horton J, et al. Society of Surgical Oncology-American Society for Radiation Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Stages I and II Invasive Breast Cancer. Int J Radiat Oncol Biol Phys. 2014 Mar 1. 88(3):553-64.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015 Jul 23
- Barclay L. New ASCO Guidelines Address HER2-Positive Breast Cancer. Medscape Medical News. Available at http://www.medscape.com/viewarticle/824751. May 7 2014; Accessed: April 6, 2017.
- Giordano SH, Temin S, Kirshner JJ, et al. Systemic Therapy for Patients With Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014 May 5.
Partridge AH, Rumble RB, Carey LA, Come SE, Davidson NE, Di Leo A, et al. Chemotherapy and Targeted Therapy for Women With Human Epidermal Growth Factor Receptor 2-Negative (or unknown) Advanced Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014 Sep 2
