EPIDEMIOLOGY
Ovarian cancer is the leading cause of death from gynecologic malignancy in the United States. In the United States each year, there are approximately 22,000 new cases of ovarian cancer and 14,000 cancer-related deaths.(1,2) Worldwide, the number of new cases of ovarian cancer each year is approaching 250,000.(3) It is the seventh most common cancer in women, and incidence rates are highest in developed countries. The incidence of ovarian cancer increases with age.
In Pakistan, ovarian cancer is the 4th most common malignancy in women. It is the most common cancer of gynecologic origin.(4,5)
PATHOPHYSIOLOGY(6)
Historically, most theories of the pathophysiology of ovarian cancer included the concept that it begins with the dedifferentiation of the cells overlying the ovary. During ovulation, these cells can be incorporated into the ovary, where they then proliferate. However, new evidence indicates that the majority of these tumors actually originate in the fimbria of the fallopian tube. Detailed pathologic studies have pushed much of the thinking about the origin of these tumors in this direction.
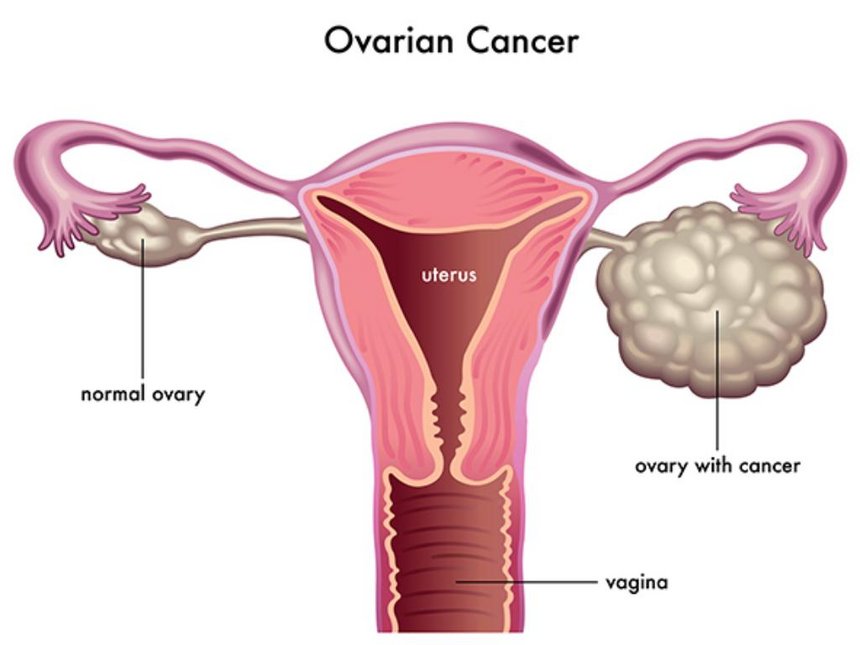
Ovarian cancer typically spreads to the peritoneal surfaces and omentum. Spread can occur by local extension, lymphatic invasion, intraperitoneal implantation, hematogenous dissemination, or transdiaphragmatic passage. Intraperitoneal dissemination is the most common and recognized characteristic of ovarian cancer. Malignant cells can implant anywhere in the peritoneal cavity but are more likely to implant in sites of stasis along the peritoneal fluid circulation.
These mechanisms of dissemination represent the rationale to conduct surgical staging, debulking surgery, and intraperitoneal administration of chemotherapy. In contrast, hematogenous spread is clinically unusual early on in the disease process, although it is not infrequent in patients with advanced disease.
Epithelial tumors represent the most common histology (90%) of ovarian tumors. Other histologies include the following:
- Sex-cord stromal tumors
- Germ cell tumors
- Primary peritoneal carcinoma
- Metastatic tumors of the ovary
Epithelial ovarian cancer
Epithelial ovarian cancer is thought to arise from epithelium covering the fimbria of the fallopian tubes, or the ovaries, both of which are derived from the coelomic epithelium in fetal development. This coelomic epithelium is also involved in formation of the müllerian ducts, from which the fallopian tubes, uterus, cervix, and upper vagina develop.
Four main histologic subtypes, which are similar to carcinoma, arise in the epithelial lining of the cervix, uterus, and fallopian tube, as follows:
- Serous (from fallopian tube)
- Endometrioid (endometrium)
- Mucinous (cervix)
- Clear cell (mesonephros)
Some variation is observed in the patterns of spread and disease distribution within the various histologic subtypes.
Epithelial tumors are found as partially cystic lesions with solid components. The surface may be smooth or covered in papillary projections, and the cysts contain fluid ranging from straw-colored to opaque brown or hemorrhagic.
Epithelial ovarian cancer most often spreads initially within the peritoneal cavity. Metastatic disease often is found on the peritoneal surfaces, particularly on the undersurface of the diaphragms, the paracolic gutters, the bladder, and the cul-de-sac. Other common sites are as follows:
- Surface of the liver
- Mesentery and serosa of the large and small bowel
- Omentum
- Uterus
- Para-aortic and pelvic lymph nodes
Outside the peritoneal cavity, epithelial ovarian cancer may spread to the pleural cavity, lungs, and groin lymph nodes. The presence of pleural effusion does not necessarily indicate disease in the chest, and malignancy can be diagnosed only cytologically. Mucinous tumors tend to form large dominant masses, while papillary serous tumors have a more diffuse distribution and are more commonly bilateral. Endometrioid and clear-cell variants more commonly exhibit local invasion, retroperitoneal disease, and hepatic metastases.
Increasing evidence suggests that a high proportion of high-grade serous carcinoma originates from distal fallopian tube epithelium or the tuboperitoneal junction rather than the ovarian surface epithelium. Serous intraepithelial or early invasive carcinoma has been found in up to 10% of fallopian tubes from BRCA mutation carriers who had undergone prophylactic bilateral salpingo-oophorectomies. Clinical, molecular, and genetic studies, as well as in vitro and animal models, have also supported a tubal origin for high-grade serous ovarian carcinoma.
Those findings have prompted the suggestion that prevention of ovarian cancer in selected women at high risk could be better accomplished with salpingectomy.
Tumors of low malignant potential
Tumors of low malignant potential (LMP), or borderline tumors, are a distinct variety of epithelial ovarian cancer that behave in a much less aggressive fashion and have a very favorable prognosis. These tumors cause great anxiety to patients, and the concept of LMP sometimes is difficult to explain. They comprise approximately 20% of malignant ovarian tumors. The mean age of diagnosis is younger than for invasive epithelial ovarian cancer, at approximately 48 years, and no large peak of incidence is observed.
These tumors are staged identically to epithelial ovarian cancer, using the FIGO (Fédération Internationale de Gynécologie et d’Obstétrique; International Federation of Obstetrics and Gynecology) system. In contrast to epithelial ovarian cancer, however, most LMP tumors are stage I at presentation, with a distribution as follows:
- Stage IA: 51%
- Stage IB: 6%
- Stage IC: 18%
- Stages II-III: 15%
- Stage IV: 2%
LMP tumors can cause a range of symptoms similar to epithelial ovarian cancer, including increasing abdominal girth, an abdominal mass, abdominal pain, abnormal uterine bleeding, urinary symptoms, and gastrointestinal symptoms. They may be asymptomatic and found on routine physical examination or ultrasound scan.
Malignant germ cell tumors
Malignant germ cell tumors (GCTs), which include dysgerminoma, endodermal sinus tumor, malignant teratoma, embryonal carcinoma, and choriocarcinoma, are thought to derive from primitive germ cells in the embryonic gonad. GCT of the ovary is much rarer than GCT of the testis in males, and much of the development of the management approach has been based on experience with male GCT.
Common characteristics of these tumors include rapid growth, a predilection for lymphatic spread, frequent mixtures of tumor types, and a predominantly unilateral pattern of ovarian involvement (except for dysgerminoma). GCT is much more common in young women but occasionally occurs in infants and older women.
Many GCTs produce tumor markers that can be measured in the blood and then used to monitor response to treatment and for follow-up care. Endodermal sinus tumors secrete alpha-fetoprotein and choriocarcinoma, and dysgerminomas occasionally secrete beta human chorionic gonadotropin (bHCG). Dysgerminoma may secrete lactate dehydrogenase and placental alkaline phosphatase.
No factors have been established related to etiology, apart from an increased incidence associated with dysgenetic gonads.
Although these tumors may be asymptomatic and present as a palpable mass, many patients present with abdominal pain. The mass may lead to acute pain due to torsion, rupture, or hemorrhage, or, patients may have abdominal distension, vaginal bleeding, or fever.
Most are stage I and confined to the ovary at the time of diagnosis.
Dysgerminoma: This is the most common malignant GCT and represents 3-5% of all ovarian malignancies. Ninety percent occur in people younger than 30 years, and 75% occur in the second and third decades, with a median age of 22 years.
Dysgerminomas are bilateral in 10-35% of cases. Five percent occur in phenotypic females with abnormal gonads. They may have a 46XY karyotype with pure gonadal dysgenesis or androgen insensitivity syndrome, or, they may have a 45X, 46XY karyotype with mixed gonadal dysgenesis. Dysgerminomas may be large and usually are solid, with a smooth external surface and a fleshy pink-tan color inside. The majority are confined to the ovary at diagnosis, but approximately 25% of otherwise stage I dysgerminomas have lymph node metastasis.
Cystic teratoma: Teratomas are germ cell tumors commonly composed of multiple cell types derived from one or more of the 3 germ layers. Inconsistent nomenclature often confuses discussions of various subtypes of teratomas. The word is derived from the Greek teras, meaning monster, which Virchow coined in the first edition of his book on tumors published in 1863. Teratomas range from benign, well-differentiated (mature) cystic lesions to those that are solid and malignant (immature). Additionally, teratomas may be monodermal and highly specialized. Rarely, within some mature teratomas certain elements (most commonly squamous components) undergo malignant transformation.
Immature teratoma: This is the second most common GCT. It occurs mostly in females aged 10-20 years but may occur after menopause. The tumor spreads most commonly to peritoneal surfaces.
Other germ cell tumors: Endodermal sinus tumor occurs at a mean age of 18 years, and one third occur before puberty. Embryonal carcinoma and choriocarcinoma are extremely rare.
Sex-cord stromal tumors
These include tumors arising from the sex cords; granulosa cells; Sertoli cells; and the specialized stroma of the genital ridge, theca, and Leydig cells. They comprise fewer than 5% of all ovarian tumors.
Although granulosa cell tumors are malignant and Sertoli-Leydig cell tumors less so, they behave in a much less malignant fashion than epithelial ovarian cancers. Benign tumors in the group include thecoma and fibroma. Granulosa cell tumors and pure Sertoli cell tumors commonly secrete estrogen, while Leydig cell tumors and combined Sertoli-Leydig tumors often secrete androgens.
Granulosa cell tumor: This is the most common malignant sex-cord stromal tumor. Ninety percent of granulosa cell tumors are stage I at the time of diagnosis. This tumor account for approximately 2% of all ovarian tumors and can be divided into adult (95%) and juvenile (5%) types based on histologic findings. Juvenile granulosa cell tumor is a variant of granulosa cell tumor that is rarely malignant. It most often presents in young girls with isosexual precocious puberty. The tumor is usually unilateral and confined to the ovary and can be managed with surgery alone.
Granulosa cell tumor can occur at any age, with a mean age of the early 50s. Because of the secretion of estrogen, the presenting features depend on the patient’s age. Prepubertal girls typically present with precocious sexual development, women of reproductive age have heavy or irregular periods, and postmenopausal women may have postmenopausal bleeding. At all ages, the tumor may present with acute abdominal pain due to rupture or hemorrhage.
Sertoli-Leydig cell tumor: These tumors are rare. They are a form of low-grade malignancy that typically produces androgens and rarely estrogens.
Other rare tumors
Small-cell carcinoma is a rare type of carcinoma that occurs in females aged 2-46 years. It often is associated with hypercalcemia.
The most common form of sarcoma in the ovary is the mixed mesodermal sarcoma or carcinosarcoma.
Metastatic tumors of the ovary arise from direct extension and spread within the bloodstream or lymphatic system or within the peritoneal cavity. Sites of origin include the endometrium; cervix; and nongynecologic sites such as breast, colon, and stomach. The classic Krukenberg tumor refers to bilateral enlargement of the ovaries from metastases from a signet-ring carcinoma of the stomach.
NATURAL HISTORY
Malignant lesions of the ovaries include primary lesions arising from normal structures within the ovary and secondary lesions from cancers arising elsewhere in the body. Primary lesions include epithelial ovarian carcinoma (70% of all ovarian malignancies), germ-cell tumors, sex-cord stromal tumors, and other more rare types. Metastases to the ovaries are relatively frequent, with the most common being from the endometrium, breast, colon, stomach, and cervix.(6)
The natural history of early ovarian cancer could alternately be explained by multifocal tumorigenesis, with multiple tumors developing simultaneously in the peritoneal epithelium or unifocal tumorigenesis when the tumor develops in the ovary and spreads to other sites. Woodruff and Julian(7) studied a large number of ovarian malignancies thought to be metastatic, but after review of histology and analysis of survival, they reclassified them as synchronous primary lesions. Russell and colleagues(8) reviewed 128 cases of primary ovarian carcinoma. They found 8 of 10 (80%) borderline carcinomas and 37 of 75 (49%) invasive serous carcinomas to have evidence of independent primary neoplasia at more than one anatomic site.
SIGN AND SYMPTOMS
History
Assessment of women for their risk of ovarian cancer necessitates obtaining a careful family history of both male and female relatives, including those relatives without cancer. If possible, obtain verification of the histologic diagnoses. The counsel of a trained geneticist is ideal. Significant problems are involved in the counseling of women and their families with regard to genetic testing and its implications. Carriers of mutations may be detected through laboratory analysis of the genetic structure of white blood cells.
Epithelial ovarian cancer presents as a wide variety of vague and nonspecific symptoms, including bloating, abdominal distension or discomfort, pressure effects on the bladder and rectum, constipation, vaginal bleeding, indigestion and acid reflux, shortness of breath, tiredness, weight loss, and early satiety. The patient may feel an abdominal mass.
Physical Examination
Physical findings are uncommon in patients with early disease. Patients with more advanced disease may present with any of the following:
- Ovarian or pelvic mass
- Ascites
- Pleural effusion
- Abdominal mass or bowel obstruction
RATIONALE FOR SCREENING
Most experts feel that a screening protocol for ovarian cancer should have a positive predictive value of at least 10 percent (that is, no more than nine healthy women with false-positive screens would undergo unnecessary procedures for each case of ovarian cancer detected).(9-11) A screening program that targets all women over age 50 would require a test with a specificity of at least 99.6 percent (assuming a sensitivity of 80 percent) to achieve a positive predictive value of 10 percent.
Tests that have been evaluated for screening in certain circumstances include measurement of the CA 125 tumor marker or other serologic markers, ultrasonography, and combinations of these modalities (multimodal screening [MMS]). Image-based screening by computed tomography (CT) scanning is unlikely to be effective in diagnosing presymptomatic early disease.(12)
DIAGNOSTIC TESTS(6)
Tumor Markers
Tumor markers are glycoproteins that are usually detected by monoclonal antibodies. Each tumor marker has a variable profile of usefulness for screening, determining diagnosis and prognosis, assessing response to therapy, and monitoring for cancer recurrence. They are produced by tumor cells in response to cancer or certain benign conditions and indicate biological changes that signal the existence of malignancy. These soluble molecules can usually be detected in elevated quantities in the blood, urine, or body tissues of patients with certain types of cancer.
Due to the location of ovarian tumors within the abdominal cavity, making a preoperative pathological diagnosis of cancer is difficult without laparotomy. From this point of view, the use of tumor markers that consist of carbohydrate antigens, such as CA125, in addition to diagnostic imaging, is useful in the diagnosis of ovarian cancer.
CA125 is a glycoprotein antigen detected by using mouse monoclonal antibody OC125 raised from an ovarian cancer cell line. CA125 is not specific for epithelial ovarian cancer and is elevated in other benign and malignant conditions, including menstruation; endometriosis; pelvic inflammation; liver, renal, and lung disease; and cancer of the endometrium, breast, colon, pancreas, lung, stomach, and liver. It is also elevated in 6% of women who do not have epithelial ovarian cancer. Although CA125 is elevated in 83% of women with epithelial ovarian cancer, it is elevated in only 50% of those with stage I disease.
A monoclonal antibody-based immunoassay for CA125 has been used to monitor the treatment of epithelial ovarian carcinomas. Persistent elevation of CA125 in serum has generally reflected persistence of disease at second-look surveillance procedures. However, CA125 levels can return to within normal limits and residual disease can be found at laparoscopy or laparotomy.
CA125 is not useful when used alone as a single one-time test for ovarian cancer screening, but it may have increased value when serial measurements are performed over time and if it is incorporated into a risk of ovarian cancer algorithm. CA125 shows promise for distinguishing benign from malignant pelvic masses.
Tests that use multiple markers have been devised. The OVA1 test includes five markers: transthyretin, apolipoprotein A1, transferrin, beta-2 macroglobulin, and CA125. The Ovasure test includes six markers: leptin, prolactin, osteopontin, insulinlike growth factor II, macrophage inhibitory factor, and CA125. The National Comprehensive Cancer Network (NCCN) does not endorse either of those for ovarian cancer screening.
Other markers that have been investigated include lysophosphatidic acid, tumor-associated glycoprotein 72 (TAG 72), OVX1, and macrophage colony-stimulating factor (M-CSF). Newer experimental markers have been identified through various laboratory techniques. These markers include mesothelin, human epididymis protein 4, kallikrein, and haptoglobin-alpha.
Imaging in Ovarian Cancer
Imaging studies used in ovarian cancer include ultrasonography, chest radiography, computed tomography (CT), and magnetic resonance imaging (MRI). Positron emission tomography (PET) scanning does not have an established role in the diagnosis of primary ovarian malignancy.
Ultrasonography is the most useful initial investigation in a patient found to have a pelvic mass. This may define the morphology of the pelvic tumor. In addition, it can determine whether large masses are present in other parts of the abdomen, including in the liver.
Chest radiography or CT is performed routinely, as it is useful in helping exclude pleural effusions or pulmonary spread of malignant diseases of the ovary.
The primary advantage of using MRI in the evaluation of ovarian masses is the ability to employ this modality in the characterization of tissue. The presence of fat, hemorrhage, mucin, fluid, and solid tissue within an ovarian mass can be determined with the aid of MRI. The ability to characterize tissue in this way is most useful in determining whether a mass is definitely benign.
In many cases, CT is complementary to surgical staging. CT can identify possible sites of unsuspected disease such as the pelvic peritoneum, paraaortic nodes, diaphragm, and chest.
GI Tract Workup
In patients with diffuse carcinomatosis and GI symptoms, a GI tract workup may be indicated, including one of the following:
- Upper and/or lower endoscopy
- Barium enema
- Upper GI series
Carcinoembryonic antigen (CEA) levels may also be measured.
Biopsy
Fine-needle aspiration (FNA) or percutaneous biopsy of an adnexal mass is not routinely recommended. In most cases, this approach may only serve to delay diagnosis and treatment of ovarian cancer. Instead, if a clinical suggestion of ovarian cancer is present, the patient should undergo a surgical evaluation for diagnosis and staging.
FNA, percutaneous biopsy, or diagnostic paracentesis should be performed in patients with diffuse carcinomatosis or ascites without an obvious ovarian mass, and in patients who will be treated with neoadjuvant chemotherapy.
Mammography
The preoperative workup also should include mammography for women older than 40 years who have not had a recent mammogram. This is especially important in women with estrogen-producing tumors because these may increase the risk of breast malignancies.
Additionally, breast cancers can metastasize to the ovaries, often bilaterally. Mammography can help rule out the possibility of a nongynecologic primary neoplasm in the breast. (19)
Staging
Ovarian cancer is typically staged by means of the system formulated and updated by the International Federation of Obstetrics and Gynecology (FIGO),(13) as listed below.
Stage I
In stage I, growth is limited to the ovaries. Substages are as follows:
- Stage IA – Tumor limited to 1 ovary, capsule intact, no tumor on external surface
- Stage IB – Tumor involves both ovaries, capsule intact, no tumor on external surface
- Stage IC – Tumor either stage IA or IB, plus surgical spill (IC1), capsule rupture before surgery or tumor on ovarian surface (IC2), or malignant cells in the ascites or peritoneal washings (IC3)
Stage II
In stage II, tumor involves one or both ovaries, with pelvic extension (below the pelvic brim) or primary peritoneal cancer. Substages are as follows:
- Stage IIA – Extension to and/or implants on the uterus or fallopian tubes
- Stage IIB – Extension to other pelvic intraperitoneal tissues
Stage III
In stage III, tumor involves one or both ovaries, with cytologically or histologically confirmed spread to the peritoneum outside the pelvis, and/or metastasis to the retroperitoneal lymph nodes. Substages are as follows:
- Stage IIIA – Positive retroperitoneal lymph nodes and/or microscopic metastasis beyond the pelvis
- Stage IIIA1 – Positive retroperitoneal lymph nodes only (IIIA1), metastasis ≤10 mm (IIIA1[ii]), or metastasis >10 mm (IIIA1[ii])
- Stage IIIA2 – Microscopic, extrapelvic (above the brim) peritoneal involvement ± positive retroperitoneal lymph nodes
- Stage IIIB – Macroscopic, extrapelvic, peritoneal metastasis ≤2 cm ± positive retroperitoneal lymph nodes; includes extension to capsule of liver/spleen
- Stage IIIC – Macroscopic, extrapelvic, peritoneal metastasis >2 cm ± positive retroperitoneal lymph nodes; includes extension to capsule of liver/spleen
Stage IV
Stage IV comprises distant metastasis, excluding peritoneal metastasis. Substages are as follows:
- Stage IVA – Pleural effusion with positive cytology
- Stage IVB – Hepatic and/or splenic parenchymal metastasis, metastasis to extra-abdominal organs (including inguinal lymph nodes and lymph nodes outside of the abdominal cavity)
Other recommendations
Additional major recommendations from FIGO include the following:
- Histologic type, including grading, should be designated at staging
- The primary site (ovary, fallopian tube, or peritoneum) should be designated if possible
- Tumors that may otherwise qualify for stage I but are involved with dense adhesions justify upgrading to stage II if tumor cells are histologically demonstrated in the adhesions
THERAPY CONSIDERATION
Surgery is often the initial treatment of choice for ovarian cancer, provided patients are medically fit. Patients who are not candidates for optimal debulking should be considered for neoadjuvant chemotherapy followed by interval debulking surgery and further chemotherapy. Patients who are not fit for surgery may be given chemotherapy and considered for surgery later, or treated primarily with chemotherapy.
Surgery should be used in conjunction with chemotherapy with a taxane and a platinum compound (e.g. paclitaxel plus carboplatin).
In women who present with peritoneal carcinomatosis but without an obvious pelvic mass, an extensive search often fails to identify a primary tumor. These patients can be presumed to have ovarian carcinoma or primary peritoneal carcinoma and can be treated with cytoreductive surgery and platinum-based chemotherapy.
TREATMENT OPTIONS(6)
Choosing Appropriate Surgery
The appropriate surgical approach varies, depending on whether disease is visible outside the ovaries. For patients with no disease visible outside the ovaries, adequate surgical staging is essential because the incidence of microscopic metastases is significant. Surgery for patients with stage IV disease should be individualized, particularly when disease is in the liver and above the diaphragm. Patients who are in stage IV because of small-volume disease in the liver, abdominal wall, or thorax can be considered for cytoreductive surgery if medically fit.
If the patient does not desire future fertility, perform a total abdominal hysterectomy and excise the opposite ovary. Appendectomy can be performed if a mucinous tumor is present.
If macroscopic disease is visible outside of the ovary, all visible tumor should be removed. This may require extensive surgery, including bowel resection, excision of peritoneal implants, liver resection, omentectomy, and splenectomy.
The extent of bowel resection should depend on the role this plays in achieving maximal cytoreduction.
Surgical Staging
The standard care for ovarian cancer includes surgical exploration for primary staging and for cytoreduction or debulking. If the disease appears to be confined to the pelvis, comprehensive surgical staging is indicated.
The surgical approach should be individualized and may involve laparotomy or use of a minimally invasive approach. Regardless of approach, staging requires several key components. Careful inspection and/or palpation of the abdominal contents should be performed, including all peritoneal surfaces, the liver, large and small bowel and mesentery, stomach, appendix, kidneys, spleen, retroperitoneal spaces, and all pelvic structures.
The staging procedure should include the following:
- Peritoneal cytology
- Multiple peritoneal biopsies
- Omentectomy
- Pelvic and para-aortic lymph node sampling.
Cytoreductive Surgery
Cytoreductive surgery should be performed by a gynecologic oncologist at the time of initial laparotomy. The volume of residual disease at the completion of surgery represents one of the most powerful prognostic factors.
According to the National Comprehensive Cancer Network (NCCN) ovarian cancer guidelines, in newly diagnosed invasive epithelial ovarian cancer that involves the pelvis and upper abdomen, residual disease of less than 1 cm is evidence of optimal cytoreduction, although the greatest possible effort should be made to remove all obvious disease.(15) The NCCN notes that one or more of the following procedures may be considered for optimal surgical cytoreduction:
- Bowel resection and/or appendectomy
- Stripping of the diaphragm or other peritoneal surfaces
- Splenectomy
- Partial cystectomy and/or ureteroneocystotomy
- Partial hepatectomy
- Partial gastrectomy
- Cholecystectomy
- Distal pancreatectomy
Patients with advanced ovarian cancer are classified in three groups as follows, based on the postoperative residual tumor:
- Good risk – Microscopic disease outside the pelvis (stage IIIa) or macroscopic disease less than 2 cm outside the pelvis (stage IIIb)
- Intermediate risk – Macroscopic disease less than 2 cm outside the pelvis only after surgery
- Poor risk – Macroscopic disease more than 2 cm after surgery or disease outside the peritoneal cavity
Interval Debulking
Interval debulking can be performed in patients whose cancer was not adequately debulked at the time of initial surgery. It should also be considered in those patients in whom an initial debulking surgery was not attempted.
Patients receive three cycles of postoperative chemotherapy. Approximately 60% of patients are then able to undergo optimal resection. Surgical treatment is followed by three more cycles of chemotherapy.
Laparoscopic Surgery
According to guidelines developed by the American College of Obstetricians and Gynecologists, laparoscopy may be used for diagnostic purposes in a patient at low risk for ovarian cancer and to remove cystic masses, provided that all the following criteria are met:(16)
- The mass is 10 cm or smaller as viewed by a sonogram
- The mass has a distinct border and no solid parts
- No associated ascites is present
- The serum CA125 level is normal (< 35 U/mL)
- The patient has no family history of ovarian cancer
If a chance exists that ovarian cancer may be present, surgery is best arranged in conjunction with a specialist in gynecologic cancer surgery. The patient can then undergo all necessary surgery for her cancer during a single anesthetic session, without delay.
As part of initial treatment of epithelial ovarian cancer, laparoscopic surgery may be performed for early-stage disease when no disease is visible outside of the ovaries. Its use in more advanced disease, when spread is visible outside the ovaries, is more limited due to the scope of cytoreductive surgery necessary and the risk of port-site recurrence. Laparoscopy also has a role in second-look inspection and in the staging of apparently early-stage disease found by chance during another surgery.
The NCCN ovarian cancer guidelines state that minimally invasive surgery may be used by an experienced surgeon in selected patients to achieve surgical staging and debulking. In addition, the NCCN considers that minimally invasive surgery may be useful when evaluating whether maximum cytoreduction can be achieved in patients with newly diagnosed or recurrent ovarian cancer.(15)
Radiation Therapy
Radiation has not been widely accepted as a routine treatment modality in the initial treatment of patients with epithelial ovarian cancer, despite reports of efficacy for higher-risk stage I and II disease and in stage III disease where small-volume residual disease is present after surgery. In selected cases, pelvic diseases may respond to palliative dosing regimens with minimal toxicity.
Estrogen Replacement Therapy
The safety of estrogen replacement therapy (ERT) after treatment for epithelial ovarian cancer has not been tested in a randomized trial, but current evidence suggests that the benefits of ERT outweigh the risks.
In younger women with endometrioid subtypes, ERT is a concern because these tumors theoretically are estrogen sensitive. If estrogen is used in such patients, a progestin should also be considered.
Treatment protocol
Treatment protocols for ovarian cancer are provided below, including the following:
- Treatment by stage
- Treatment of patients with implants
- Consolidation therapy
- Treatment of recurrent disease
General treatment approach(14)
Surgery is the initial modality of treatment for stage I-IVA epithelial ovarian cancer. Only a small percentage of women with epithelial ovarian cancer can be treated with surgery alone. This small percentage includes patients with stage IA (grade 1) and stage IB (grade 1) serous, mucinous, endometrioid, and Brenner tumors. The treatment of stage 1C, all grades, and stage IA and IB grade 2 and 3 is nevertheless controversial.
Clear-cell carcinomas are associated with a significantly worse prognosis in stage I; all patients with this histologic subtype should be considered for chemotherapy.
Chemotherapy recommendations based on stage
Stage I:
Consider chemotherapy for stages 1A and 1B grades 2 and 3, and stage 1C. Chemotherapy is usually given after surgery. Regimens are as follows:
- Paclitaxel 175 mg/m2 IV over 3 h plus carboplatin area under the curve (AUC) 5-6 IV over 1 h on day 1; every 21 d for three to six cycles or
- Docetaxel 60-75 mg/m2 IV over 1 h plus carboplatin AUC 5-6 IV over 1 h on day 1; every 21 d for three to six cycles or
- Carboplatin AUC 5 IV over 1 h on day 1 plus pegylated liposomal doxorubicin 30 mg/m2 IV up to 1 h; every 28 d for three to six cycles
Stages IIA and IIB
All patients with stage II or higher cancer should be considered for front-line chemotherapy based on burden of disease and ability to achieve optimal primary resection.
- Paclitaxel 175 mg/m2 IV over 3 h plus carboplatin AUC 5-6 IV over 30 min on day 1; every 21 d for three to six cycles or
- Dose-dense paclitaxel 80 mg/m2 IV over 1 hr on days 1, 8, and 15 plus carboplatin AUC 5-6 IV over 1 h on day 1; every 21 d for six cycles or
- Paclitaxel 60 mg/m2 IV over 1 h followed by carboplatin AUC 2 IV over 30 min; weekly for 18 weeks or
- Docetaxel 60-75 mg/m2 IV over 1 h plus carboplatin AUC 5-6 IV over 1 h on day 1; every 21 d for three to six cycles or
- Carboplatin AUC 5 IV over 1 h plus pegylated liposomal doxorubicin 30 mg/m2; every 28 d for six cycles
Stage III
At this time, there is no standardized regimen for Intraperitoneal (IP) therapy; however, the following dosing regimens may be used:
- Paclitaxel 135 mg/m2 continuous IV infusion over 3 h or 24 h on day 1 plus cisplatin 75-100 mg/m2 IP on day 2 (may reduce dose to 75 mg/m2) plus paclitaxel 60 mg/m2 IP on day 8; repeat every 3 weeks for si6 cycles, provided that the disease is responsive
- The cisplatin dose may be reduced to 75 mg/m2 IP on day 2; some clinicians give paclitaxel 135 mg/m2 IV over 3 h followed by cisplatin 75 mg/m2 IP, both on day 1 and on an outpatient basis
- Normal range of carboplatin AUC for treatment of ovarian carcinoma is from 5 to 7.5; patients who have received extensive prior chemotherapy or radiation should start with an AUC < 5
If the patient cannot tolerate IP delivery, revert to one of the following two drug regimens:
- Paclitaxel 175 mg/m2 IV over 3 h plus carboplatin AUC 5-6 IV over 1 h on day 1; every 21 d for six cycles or
- Docetaxel 75 mg/m2 IV over 1 h plus carboplatin AUC 5 IV over 1 h on day 1; every 21 d for six cycles
Stage IVA:
Treatment recommendations are similar to those for stage III.
Stage IVB:
Possible regimens are as follows:
- Paclitaxel 175 mg/m2 IV over 3 h plus carboplatin AUC 5-6 IV over 1 h on day 1; every 21 d for six cycles or
- Docetaxel 75 mg/m2 IV over 1 h plus carboplatin AUC 5 IV over 1 h on day 1; every 21 d for six cycles
Neoadjuvant Chemotherapy
While neoadjuvant chemotherapy has been demonstrated as noninferior to primary cytoreductive surgery and could be considered in all patients with advanced disease, it may be of particular benefit in certain subgroups of patients. Women who are unlikely to achieve optimal up-front surgical debulking due to extensive disease (lung or liver metastasis, disease in the porta hepatis, significant disease in the small bowel mesentery, or massive ascites) or those who are poor candidates to withstand aggressive surgery should be considered for neoadjuvant chemotherapy.
Regimens include the following:
- Paclitaxel 175 mg/m2 IV over 3 h plus carboplatin in AUC 5-6 IV over 1 h on day 1; every 21 d or
- Docetaxel 60-75 mg/m2 IV over 1 h plus carboplatin AUC 5 IV over 1 h on day 1; every 21 d
GOALS OF THERAPY
The overall goal of cancer treatment is to significantly decrease the number of deaths, continuously improve early detection.
The goal of treatment for ovarian cancer is to surgically remove as much of the cancer as possible (called “debulking” or “cytoreductive surgery”). The aim of treatment will be to put the tumour into remission, so it shrinks or disappears and then to provide adjuvant, or additional therapy, such as chemotherapy, to kill any possibly remaining cancer cells in the body. Radiation therapy, which uses high energy rays to kill cancer cells, is not typically utilized in ovarian cancer.
GUIDE LINES
To view, “NCCN Guidelines for Ovarian Cancer 2017”, please click on below link:
https://www2.tri-kobe.org/nccn/guideline/gynecological/english/ovarian.pdf
To view, “Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up”, please click on below link:
https://www.esmo.org/Guidelines/Gynaecological-Cancers/Newly-Diagnosed-and-Relapsed-Epithelial-Ovarian-Carcinoma/eUpdate-Treatment-Recommendations
To view, “Non-Epithelial Ovarian Cancer: ESMO Clinical Practice Guidelines 2018”, please click on below link:
https://www.esmo.org/Guidelines/Gynaecological-Cancers/Non-Epithelial-Ovarian-Cancer
To view, “U.S. Preventive Services Task Force (USPSTF) – Ovarian cancer screening guidelines”, please click on below link:
https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/ovarian-cancer-screening
CONSULTATION AND LONG TERM MONITORING
Consult a gynecologic oncologist if ovarian cancer is suspected. The question of when to obtain preoperative consultation with a gynecologic oncologist can be difficult to delineate. A good rule of thumb is that all postmenopausal and premenarchal patients with adnexal masses should have the benefit of a consultation with an oncologist, because the risk of malignancy is greater. In reproductive-aged patients, the vast majority of adnexal masses are benign.
Patients with radiologic or sonographic findings suggestive of malignancy (e.g. solid or mixed solid and cystic tumors, ascites) and patients with endocrinologic symptoms and an adnexal mass should have the benefit of a preoperative consultation with a gynecologic oncologist. Patients with a question of malignancy preoperatively can also be evaluated with serum tumor markers including CA125, CA19-9, lactate dehydrogenase (LDH), AFP, beta-hCG, and inhibin levels. Appropriate referral should be made if any of these are significantly elevated.
Patients with primarily gastrointestinal (GI) complaints may benefit from a consultation with a gastroenterologist to rule out a primary GI source prior to surgical exploration. Endoscopy can be performed during this preoperative evaluation if indicated.
Follow-up should occur at 2- to 3-month intervals for the first 2 years for patients not undergoing chemotherapy. Then, this can be spaced out to every 4-6 months for the next 3 years, then yearly thereafter. A history should be obtained and pelvic examination should be performed at each visit. Also, serum determination of tumor markers should be performed if these were elevated preoperatively or immediately postoperatively.
If any evidence of recurrence arises during follow-up, imaging studies, usually an abdominopelvic CT scan should be performed to look for recurrent tumors. Most recurrences are confined to the abdomen and pelvis. Other imaging studies may be ordered as dictated by physical examination findings.
PRECAUTIONS
At present, there is no known method to prevent ovarian cancer, but some things appear to reduce a woman’s risk of developing the disease. They include:
- Oral contraception: Birth control pills reduce the risk of ovarian cancer especially among women who use them for several years. Compared with women who never used oral contraceptive, those who used oral contraceptives for 3 years or more have about a 30%-50% lower risk of developing ovarian cancer.
- Breast feeding and pregnancy: Having one or more children, particularly if the first is born before age 25, and breast feeding may decrease a woman’s risk.
- Tubal ligation: This is a surgical procedure in which the fallopian tubes are tied to prevent pregnancy. This procedure reduces the relative risk of developing ovarian cancer.
- Hysterectomy: A Hysterectomy has been demonstrated to reduce the relative risk of ovarian cancer. A woman should not have a hysterectomy exclusively to avoid the risk of ovarian cancer, but if one is being performed for valid medical reasons and she has a family history of ovarian or breast cancer or is over age forty, she should discuss concurrent ovary removal with her physician.
- Prophylactic oophorectomy: Oophorectomy is the surgical removal of one or both ovaries. Only recommended for certain high-risk patients, the operation eliminates the risk for ovarian cancer, but not the risk for a less common cancer called Primary Peritoneal Carcinoma. This cancer is similar to ovarian cancer in spread, presentation and treatment. Discussion with your physician is necessary to determine your individual risk and options for prophylactic surgery.
REFERENCES
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68:7.
- US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Screening for Ovarian Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319:588.
- World cancer research fund international. Cancer facts and figures. http://www.wcrf.org/int/cancer-facts-figures/worldwide-data (Accessed on April 17, 2017).
- Malik 1, Khan W, Khan Z. Pattern of malignant tumors observed in a University hospital: A retrospective analysis. J. l’ak. Med. Assoc., 1998;48: 120-122.
- Bhurgri Y, Bhurgri A, Hassan SH, et at. Cancer incidence in Karachi, Pakistan: first results from Karachi Cancer Registry Int. J. Cancer, 2000;85:325-29.
- Andrew E Green. Ovarian cancer. Medscape. Available from: https://emedicine.medscape.com/article/255771-overview#a3 (Accessed on 2019 Jan 24)
- Woodruff JD, Julian CG. Multiple malignancy in the upper genital tract. Am J Obstet Gynecol 1969;193:810
- Russell P, Bannatyne PM, Solomon HJ et al. Multifocal tumorigenesis in the upper female genital tract: Implications for staging and management. Int J Gynecol Pathol 1985;4:192
- Jacobs I. Genetic, biochemical, and multimodal approaches to screening for ovarian cancer. Gynecol Oncol 1994; 55:S22.
- Rosenthal AN, Menon U, Jacobs IJ. Screening for ovarian cancer. Clin Obstet Gynecol 2006; 49:433.
- Moore RG, MacLaughlan S, Bast RC Jr. Current state of biomarker development for clinical application in epithelial ovarian cancer. Gynecol Oncol 2010; 116:240.
- Pickhardt PJ, Hanson ME. Incidental adnexal masses detected at low-dose unenhanced CT in asymptomatic women age 50 and older: implications for clinical management and ovarian cancer screening. Radiology 2010; 257:144.
- Zeppernick F, Meinhold-Heerlein I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Arch Gynecol Obstet. 2014 Nov. 290 (5):839-42
- Shannon M Grabosch. Ovarian Cancer Treatment Protocols. Medscape. Available from: https://emedicine.medscape.com/article/2006723-overview (Accessed on 2019 Jan 25)
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer. Available at http://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Version 4.2017 — November 9, 2017; Accessed: January 5, 2018.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Management of adnexal masses. Obstet Gynecol. 2007 Jul. 110 (1):201-14
