EPIDEMIOLOGY
Prostate cancer is the second most common cancer in men worldwide, with over 1.2 million cases and 358,000 deaths annually, according to data from the World Health Organization (WHO) GLOBOCAN database.(1) A man’s life time risk of this disease is one out of seven.(2) Internationally, the incidence of prostate cancer varies by more than 50-fold, with the highest rates being in North America, Australia, and northern and central Europe and the lowest rates being in southeastern and south-central Asia and northern Africa.(3)
During 1998-2002, prostate cancer was the fourth common malignancy among males in Karachi (Pakistan) with an age standardized incidence rate was 10.1 per 100,000 men whereas mean age of the cases were 67.4 years.(4) According to another report, estimated incidence of prostate cancer in 2012 is 3,014 with mortality rate of 2,356.(5)
PATHOPHYSIOLOGY(6)
Prostate cancer develops when the rates of cell division and cell death are no longer equal, leading to uncontrolled tumor growth. Following the initial transformation event, further mutations of a multitude of genes, including the genes for p53 and retinoblastoma, can lead to tumor progression and metastasis. Most prostate cancers (95%) are adenocarcinomas
Approximately 4% of cases of prostate cancer have transitional cell morphology and are thought to arise from the urothelial lining of the prostatic urethra. The few cases that have neuroendocrine morphology are believed to arise from the neuroendocrine stem cells normally present in the prostate or from aberrant differentiation programs during cell transformation.
Squamous cell carcinomas constitute less than 1% of all prostate carcinomas. In many cases, prostate carcinomas with squamous differentiation arise after radiation or hormone treatment.
Of prostate cancer cases, 70% arise in the peripheral zone, 15-20% arises in the central zone, and 10-15% arises in the transitional zone. Most prostate cancers are multifocal, with synchronous involvement of multiple zones of the prostate, which may be due to clonal and nonclonal tumors.
Local spread and metastasis
When these cancers are locally invasive, the transitional-zone tumors spread to the bladder neck, while the peripheral-zone tumors extend into the ejaculatory ducts and seminal vesicles. Penetration through the prostatic capsule and along the perineural or vascular spaces occurs relatively late.
The mechanism for distant metastasis is poorly understood. The cancer spreads to bone early, often without significant lymphadenopathy. Currently, 2 predominant theories have been proposed for spread: the mechanical theory and the seed-and-soil theory.
The mechanical theory attributes metastasis to direct spread through the lymphatics and venous spaces into the lower lumbar spine. Advocates of the seed-and-soil theory, however, believe that tissue factors that allow for preferential growth in certain tissues, such as bone, must be present. Lung, liver, and adrenal metastases have also been documented. Specific tissue growth factors and extracellular matrices are possible examples.
The doubling time in early stage disease is variable. In the majority of cases, doubling time is longer than 4 years. Only a small percentage of prostate cancers double in less than 2 years. Doubling time tends to accelerate as the tumor grows and becomes more aggressive. Larger tumors usually have a higher Gleason grade and a faster doubling time.
NATURAL HISTORY(7)
Although the natural history of prostate cancer has not been fully elucidated, it is thought to arise from damaged prostate epithelium and progressively develop over many decades. Prostate disease is heterogeneous and multifocal, further complicating the understanding of its progression. Based on autopsy studies, about one-third of men over the age of 50 years display histological evidence of prostate cancer. However, a majority of these cases remain clinically insignificant, underscoring the variability in prostate cancer and the protracted nature of this disease.
The likelihood of disease progression of prostate cancer is difficult to predict. Detection of cancer from a biopsy can result in a localised diagnosis; however, upon a prostatectomy, it may be revealed that the disease had grown outside the margins of the gland or even had metastasised. Conversely, certain men diagnosed with prostate cancer may live out their natural lives without suffering any morbidity or mortality from the disease. Therefore, it becomes imperative to determine whether or not a particular lesion will stay localised or spread beyond the confines of the gland. The usually slow progression of prostate cancer allows delaying or avoiding definitive treatment (active surveillance) in selected patients if some prerequisites are fulfilled. The younger a candidate is for active surveillance, the more strict the tumour‐related criteria that should be used.
Research has revealed insights into the likely progression of prostate tumours. It has been shown that certain high‐grade tumours proceed on a more aggressive course than low‐grade, well‐differentiated tumours and therefore should be managed accordingly. The Gleason score is one of the most powerful prognostic factors in prostate cancer. In elderly patients with clinically localised, conservatively managed prostate cancer, the probability to survive the disease for at least 10 years ranges from 77% to 98% when the Gleason score is 7 or less, whereas this rate is only 33–75% in patients with a Gleason score of 8–10. The prolonged nature of prostate cancer progression highlights the opportunities for clinical therapeutic interventions that could reduce the risk of disease development and slow it or treat the existing disease.
SIGN AND SYMPTOMS(6)
Currently, the majority of prostate cancers are identified in patients who are asymptomatic. Diagnosis in such cases is based on abnormalities in a screening prostate-specific antigen (PSA) level or findings on digital rectal examination (DRE). In addition, prostate cancer can be an incidental pathologic finding when tissue is removed during transurethral resection to manage obstructive symptoms from benign prostatic hyperplasia.
Symptoms of local disease
In the pre-PSA era, patients with prostate cancer commonly presented with symptoms that included urinary complaints or retention, back pain, and hematuria. Currently, with PSA screening, most prostate cancers are diagnosed at an asymptomatic stage. When symptoms do occur, diseases other than prostate cancer may be the cause. For example, urinary frequency, urinary urgency, and decreased urine stream often result from benign prostatic hyperplasia.
Symptoms of advanced disease
Advanced prostate cancer results from any combination of lymphatic, hematogenous, or contiguous local spread. Skeletal manifestations are especially common, because prostate cancer has a strong predilection for metastasizing to bone.
Manifestations of metastatic and advanced prostate cancer may include the following:
- Weight loss and loss of appetite
- Anemia
- Bone pain, with or without pathologic fracture
- Neurologic deficits from spinal cord compression
- Lower extremity pain and edema due to obstruction of venous and lymphatic tributaries by nodal metastasis
Uremic symptoms can occur from urethral obstruction caused by local prostate growth or retroperitoneal adenopathy secondary to nodal metastasis.
RATIONALE FOR SECREENING
Digital Rectal Exam (DRE) and Prostate-Specific Antigen (PSA) evaluation are the two components used in prostate cancer screening. Transrectal ultrasonography (TRUS) has been associated with a high false-positive rate, making it unsuitable as a screening tool, although it has an established role in directing prostatic biopsies.
National Comprehensive Cancer Network guidelines
The NCCN recommends performing a baseline evaluation, with a history and physical examination that includes the following: (8)
- Family history
- Medications
- History of prostate disease and screening, including prior PSA and/or isoforms, exams, and biopsies
- Race
- Family or personal history of BRC1/2 mutations
The clinician should then discuss of the risks and benefits of a baseline PSA test with the patient, and consider a baseline DRE to identify high-risk cancers associated with a seemingly normal PSA. In patients 45-75 years of age, subsequent evaluation is based on the results of those tests, as follows:(8)
- PSA < 1 ng/mL, DRE normal (if done): Repeat testing at 2–4 year intervals
- PSA 1-3 ng/mL, DRE normal (if done): Repeat testing at 1–2 year intervals
- PSA >3 ng/mL or very suspicous DRE result: Evaluate for biopsy
Although very few men above the age of 75 years benefit from PSA testing, screening may be cautiously considered in selected cases of very healthy men with little or no comorbidity. If PSA is measured and is < 4 ng/mL, the DRE is normal (if done), and no other indications for biopsy are present, the NCCN recommends repeat testing in selected patients at 1–4 year intervals. If the PSA is >4 ng/mL or DRE results are very suspicious, the patient should be evaluated for biopsy.
The NCCN notes that men 60 years of age and older whose serum PSA is less than 1.0 ng/mL have a very low risk of metastasis or death from prostate cancer and may not benefit from further testing. The same is true of men age 75 years with a PSA of less than 3.0 ng/mL.
Evaluation for biopsy includes the following:
- Repeat PSA
- Perform DRE, if not done performed during initial risk assessment
- Workup for benign disease
DIAGNOSTIC TESTS(6)
Currently, most cases of prostate cancer are identified by screening in asymptomatic men. Digital rectal examination (DRE) and prostate-specific antigen (PSA) evaluation are the two components used in prostate cancer screening. Transrectal ultrasonography (TRUS) has been associated with a high false-positive rate, making it unsuitable as a screening tool, but it has an established role in directing prostatic biopsies.
Needle biopsy of the prostate is indicated for tissue diagnosis in patients whose screening shows elevated PSA levels or abnormal DRE findings. Pathologic evaluation of the biopsy specimen permits calculation of the Gleason score, which is used to help determine prognosis.
Blood studies beyond PSA offer little useful information for men with newly diagnosed early stage prostate cancer. For men with advanced disease, a chemistry profile (including serum creatinine and liver function tests) is warranted. Acid and alkaline phosphatase measurements do not provide helpful information in most cases.
Urinalysis should be performed. If results are abnormal (i.e. indicating the presence of hematuria or infection), further workup is warranted before planning cancer therapy.
Computed tomography (CT) scanning is rarely helpful except in men who are at high risk for lymph node metastases or who are going to be treated with radiation. Chest radiography is no longer a routinely advisable staging test for prostate cancer.
Prostate-Specific Antigen
When PSA testing was first developed, the upper limit of normal for PSA was thought to be 4 ng/mL. However, subsequent studies have shown that no PSA level guarantees the absence of prostate cancer. As the PSA level increases, so does the risk of this disease. When the PSA is 1 ng/mL, cancer can be detected in about 8% of men if a biopsy is performed. With a PSA level of 4-10 ng/mL, the likelihood of finding prostate cancer is about 25%; with a level above 10 ng/mL, the likelihood is much higher.(9)
Although cancer may be present even when the PSA level is less than 1 ng/mL, experts do not recommend a biopsy unless the PSA is higher. Some use 2.5 ng/mL as the cutoff, while others wait until it is 3 ng/mL or greater.
The European Randomized Study of Screening for Prostate Cancer (ERSPC) applied a PSA cutoff value of 3 ng/mL or higher as an indication for lateralized sextant biopsy.(10) For men with an initial PSA value of less than 3 ng/mL, the risk of developing aggressive prostate cancer and death has been found to significantly increase with PSA values in the 2-2.9 ng/mL range, although the overall risk of aggressive prostate cancer–related death remains limited.(11)
PSA velocity
PSA velocity is an important concept. To calculate velocity, at least 3 consecutive measurements on specimens drawn over at least 18-24 months should be used. Guidelines from the NCCN suggest that PSA velocity be considered in the context of the PSA level. The following PSA velocities are suspicious for cancer:(8)
PSA velocity of 0.35 ng/mL/y, when the PSA is ≤2.5 ng/mL
PSA velocity of 0.75 ng/mL/y, when the PSA is 4–10 ng/mL
Bound versus free PSA
The measurement of bound and free PSA can help to differentiate mildly elevated PSA levels due to cancer from elevated levels due to benign prostatic hyperplasia. Free PSA is calculated as a percentage of total PSA; the lower the percentage of free PSA, the higher the likelihood of cancer. For example, cancer is found at prostate biopsy in only 8% of men with greater than 25% free PSA, but in more than half of those with less than 10% free PSA.
The percentage of free PSA is generally used as an additional factor in making an informed recommendation for or against biopsy in patients with a PSA level of 4-10 ng/mL. Typically, a free PSA above 25% is considered normal. Some experts recommend biopsy when the free PSA is less than 18%; others advise a cutoff of 12%.
Free PSA percentage is most useful in men with very large glands and in patients in whom 1 biopsy result has already been negative. In healthy men with a PSA level of 4-10 ng/mL, many experts recommend biopsy without the additional free-PSA test. A common practice is to offer a course of antibiotics and anti-inflammatory drugs for a period of time and to then repeat the test to see if this treatment lowers the PSA level. However, no well-performed studies have established the value of this approach.
Prostate Biopsy
Needle biopsy of the prostate is indicated for tissue diagnosis in patients who present with elevated PSA levels or abnormal DRE findings. Transrectal ultrasonography (TRUS) is used to guide the biopsy, although MRI is being studied as an alternative. TRUS also permits measurement of the volume of the prostate. Hypoechoic areas on TRUS are commonly associated with cancers, but this finding is not specific enough for diagnostic purposes.
In patients with a persistently elevated PSA level in the face of negative biopsy results, the literature supports repeating the biopsy once or twice. Repeat biopsies may also include cores from the transition zone, which is not usually the case in initial biopsies.
Patients should receive prophylactic antibiotics at least 20 minutes before prostate biopsy, to reduce the risk of infection. No studies have proved, however, that more than 1 or 2 doses of antibiotic offer advantages over longer-term use following biopsy. Serious infections with resistant organisms are increasing.
Patient comfort is greatly improved by injecting a local anesthetic into the nerves near the seminal vesicles, under ultrasonographic guidance. An enema is usually given prior to the biopsy.
Complications: The National Cancer Institute (NCI) has observed that prostatic biopsies can have complications, including fever, pain, hematospermia, hematuria, positive urine cultures, and (rarely) sepsis. Also, the screening process itself can lead to adverse psychological effects in men who have a prostate biopsy but do not have identified prostate cancer.(12) These risks are multiplied because a significant percentage of men will have more than 1 biopsy before cancer is detected or they are considered free of the disease.
Histologic Findings
Although the change in glandular architecture represented by the Gleason score is currently the most widely used histologic parameter, it is not the only histologic change that can be observed in prostate cancers. Indeed, notable changes in cell and nuclear morphology, DNA ploidy, neuroendocrine differentiation, and vascularity can be observed and may have prognostic significance.
There is a continuum from normal prostatic epithelium to invasive carcinoma. Precursor lesions to carcinoma may include prostatic intraepithelial neoplasia (PIN) and atypical small acinar proliferation (ASAP).
The architecture of the gland remains normal but the epithelial layers become multi-layered and crowded. At a cellular level, the nucleus becomes large and nucleoli are visible. The term PIN is becoming less used in favor of atypical small acinar proliferation (ASAP), which is proliferation of usually small acini with features suggestive of but not diagnostic of cancer.
Selection Criteria for Imaging Studies
Imaging studies can be a valuable part of pretreatment staging of prostate cancer, helping to differentiate clinically localized disease (i.e. stage T1 or T2), which is generally amenable to local therapy, from more advanced disease that may require multimodal therapy. The currently accepted staging system allows for inclusion of these studies in the assessment of disease, although the medical literature suggests that no single study can be used to reproducibly detect non–organ-confined disease.
Comparisons of the accuracy of each imaging modality fail to provide a uniform consensus regarding the optimum study. For T staging, MRI is more accurate than CT scanning. Unfortunately, a significantly high inaccuracy rate limits the value of both of these tests. For example, if the test suggests extracapsular disease but the false-positive rate is about 30%, many men may be incorrectly advised to avoid prostatectomy.
MRI and CT scanning have equivalent accuracy for N staging. Neither is worthwhile unless the risk of nodal metastases is at least 15% or higher.
For M staging, bone scintigraphy with technetium-99m (99m Tc) is typically used. It is rarely indicated unless the PSA is above 10 or 20 ng/mL, however, because the likelihood of finding metastases is otherwise very low. Obtaining a baseline bone scan for aid in evaluating future tests makes little sense.
The American College of Radiology (ACR) has developed guidelines for the selection of imaging studies for pretreatment staging.(13) These guidelines rate the appropriateness of particular imaging modalities on the basis of such findings as T stage, Gleason score, PSA level, and percentage of positive biopsy cores.
ProstaScint scan: ProstaScint scans are a form of immunoscintigraphy that involves a murine monoclonal antibody that reacts with prostate-specific membrane antigen to identify cancer in the prostate and in metastatic deposits. ProstaScint scans frequently yield false-negative results, but the specificity of the study may be improved when combined with single-photon emission CT (SPECT) imaging or CT scanning.
The ACR suggests that routine use of ProstaScint scanning as an initial staging procedure is not currently justified. Some evidence supports its use in patients with postoperative treatment failure, especially to guide decisions regarding radiation therapy,(13) but no long-term studies have demonstrated the therapeutic benefits of administering salvage radiation based on ProstaScint scan results.
TNM Staging System
The tumor node metastases (TNM) staging system of the American Joint Committee on Cancer (AJCC) is used to stage prostate cancer. The current revision of the AJCC system, which took effect in January 2018, also uses both the Gleason score and the grade group for staging.(14)
T (primary tumor)
Clinical (cT) stages of primary tumor are as follows:
TX – Primary tumor cannot be assessed
T0 – No evidence of primary tumor
T1 – Clinically inapparent tumor not palpable or visible by imaging
T1a – Tumor incidental histologic finding in ≤5% of tissue resected
T1b – Tumor incidental histologic finding in >5% of tissue resected
T1c – Tumor identified by needle biopsy (because of elevated PSA level); tumors found in 1 or both lobes by needle biopsy but not palpable on digital rectal examination or reliably visible by imaging
T2 – Tumor confined within prostate (includes invasion into the prostatic apex or invasion into, but not beyond, the prostatic capsule)
T2a Tumor involves one-half or less of 1 lobe
T2b – Tumor involving more than half of a lobe but not more than 1 lobe
T2c – Tumor involving both lobes
T3 – Tumor extending through the prostatic capsule
T3a – Extracapsular extension (unilateral or bilateral)
T3b – Tumor invading seminal vesicle(s)
T4 – Tumor fixed to or invading adjacent structures other than seminal vesicles (eg, bladder neck, external sphincter, rectum, levator muscles, pelvic wall)
Pathologic (pT) stages of the primary tumor are as follows (there is no pT1 classification):
pT2– Organ confined
pT3 – Extraprostatic extension
pT3a – Extraprostatic extension or microscopic invasion of the bladder neck
pT3b – Seminal vesicle invasion
pT4 – Tumor is fixed or invades adjacent structures other than the seminal vesicles (eg, bladder, rectum)
N (nodes)
Clinical (cN) nodal stages are as follows:
NX – Regional lymph nodes were not assessed
N0 – No regional lymph node metastasis
N1 – Metastasis in regional lymph node or nodes
Regional lymph nodes are assessed via surgical removal or biopsy of the pelvic lymph nodes, including the obturator chain.
Pathologic (pN) nodal stages are as follows:
pNX – Regional nodes not sampled
pN0 – No positive regional nodes
pN1 – Metastases in regional nodes(s)
M (metastasis)
Metastatic stages are as follows:
M0 – No distant metastasis
M1 – Distant metastasis
M1a – Nonregional lymph node(s)
M1b – Bone(s)
M1c – Other site(s), with or without bone disease
Gleason Score
The Gleason grading system is used to help determine prognosis in prostate cancer. It is based on histologic evaluation of tumor biopsy specimens.
Grades are based on the extent to which the epithelium has a normal glandular structure. Grades progress from less malignant to more malignant; a grade of 1 indicates a near-normal pattern, and grade 5 indicates the absence of any glandular pattern. This scheme of grading histologic features greatly depends on the skill and experience of the pathologist and is subject to some degree of individual variation.
The predominant pattern and the second most common pattern are given grades from 1-5 (see the images below). The sum of these 2 grades is referred to as the Gleason score. Scoring based on the 2 most common patterns is an attempt to factor in the considerable heterogeneity within cases of prostate cancer. In addition, this scoring method has been found to be superior for predicting disease outcomes, compared with using the individual grades alone.
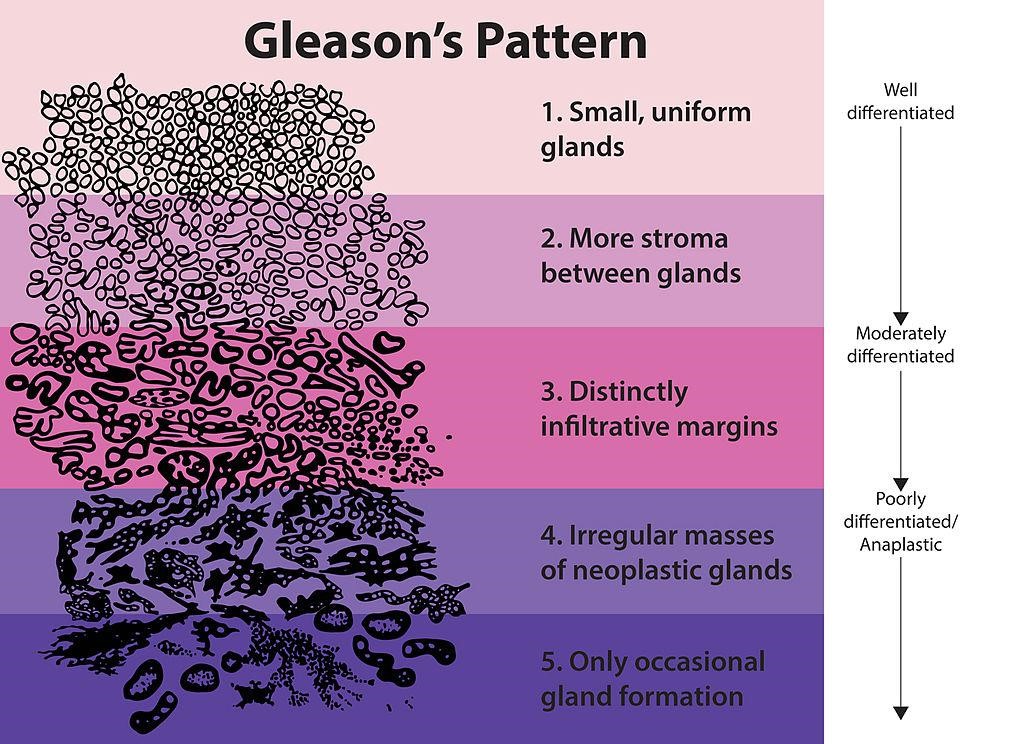
The standard approach for grading prostate cancer
The standard approach for grading prostate cancer depends on a Gleason score, which is based on pathologic evaluation of a prostatectomy specimen and is commonly estimated from prostate biopsy tissue. Prostate cancer patterns are assigned a number from 1-5; the score is created by adding the most common pattern and the highest-grade patterns.
Gleason score evaluations have changed considerably in recent years. Scores of 2-5 are rarely seen, while Gleason 7 is being reported more often.
The significance of the Gleason score is as follows:
- A score of 2-6 indicates a low-grade or well-differentiated tumor
- A score of 7 indicates a moderate-grade or moderately differentiated tumor
- A score of 8-10 indicates a high-grade or poorly differentiated tumor
Surgical Staging
In patients undergoing radical prostatectomy, pathologic assessment of extracapsular disease extension and regional nodal involvement is readily available. Routine node dissection is not worthwhile unless the likelihood of finding disease is more than 15%, which can be determined from the Partin tables.
One approach is to perform pelvic lymph node dissection selectively, limiting the procedure to men at increased risk and performing a more extensive dissection in those men. Dissected lymph nodes are sent for frozen sections; if these disclose no metastasis, the surgeon proceeds to prostatectomy.
Performing pelvic lymph node dissection in men scheduled for radiation therapy may be warranted. However, studies have shown a benefit to radiating the pelvic nodes along with the prostate, so lymph node dissection is rarely done unless a prostatectomy is planned.
THERAPY CONSIDERATION
Current guidelines on localized prostate cancer from the American Urological Association (AUA) strongly recommend that selection of a management strategy incorporate shared decision making and explicitly consider the following:(15)
- Cancer severity (risk category)
- Patient values and preferences
- Life expectancy
- Pretreatment general functional and genitourinary symptoms
- Expected post-treatment functional status
- Potential for salvage treatment
Standard treatments for clinically localized prostate cancer include the following:
- Active surveillance
- Watchful waiting
- Radical prostatectomy
- Radiation therapy
- Hormone therapy
TREATMENT OPTIONS
Localized Prostate Cancer
Whether one of the several different modalities used for treating localized prostate cancer offers survival benefits over the others remains controversial. The choice of definitive therapy has been suggested to make a significant difference in long-term survival in less than 10% of patients.
This means that most patients are cured either because the treatment was effective or because they had a non–life-threatening tumor and the treatment was unnecessary. The remainder of patients are not cured, either because they had unsuspected micrometastases or because the local therapy did not eradicate all of the malignant cells.
Current AUA guidelines (2017) consider active surveillance, radiation therapy, and radical prostatectomy to be acceptable treatment options for localized prostate cancer. However, the guidelines do not recommend any one of these therapies over the others. Instead, they advise that patients be informed of the benefits and drawbacks to the most commonly accepted interventions.(15)
The AUA guidelines emphasize that high-risk treatment should never be administered to low-risk patients. First-line hormone therapy is seldom indicated in patients with localized prostate cancer.
Intermediate-risk disease: In patients with intermediate-risk localized prostate cancer, appropriate treatment options include active surveillance, interstitial prostate brachytherapy, external beam radiation therapy, and radical prostatectomy. Cryotherapy should also be discussed. Treatment should be based in part on the patient’s preferences and functional status. A patient who chooses conventional external beam radiation therapy may have improved survival by combining it with 6 months of hormone therapy.(15)
Active surveillance: Active surveillance differs from watchful waiting. With watchful waiting, patients forgo close follow-up and primary treatment. Instead, palliative treatment is provided if local or metastatic progression occurs, as indicated by symptoms.
With active surveillance, the physician monitors the course of the disease over time and intervenes with treatment if the disease begins to progress.
Active surveillance is increasingly being recommended for men with very-low-risk disease (i.e. T1c, 2 or fewer biopsy cores positive, no core with >50% involved, Gleason 3+3/grade group 1, and a PSA density < 0.15 ng/mL/g) or low-risk disease (T1-2a disease, a Gleason score of 2-6, and a PSA level below 10 ng/mL. The National Comprehensive Cancer Network (NCCN) notes that active surveillance is usually appropriate for men with very-low-risk and low-risk prostate cancer who have a life expectancy of 10 years or more.(16)
Current NCCN recommendations for active surveillance (based on lower-level evidence) include the following:(17)
- PSA no more often than every 6 mo unless clinically indicated
- DRE no more often than every 12 mo unless clinically indicated
- Repeat prostate biopsy no more often than every 12 mo unless clinically indicated
However, the NCCN recommends a repeat biopsy within 6 months of diagnosis if the initial biopsy included fewer than 10 cores. Repeat biopsies are not indicated in patients whose life expectancy is less than 10 years.(17)
Watchful waiting: Watchful waiting is typically recommended to patients of advanced age and to those who have significant, life-limiting comorbidities or a life expectancy of less than 10 years. These patients will most likely experience worse quality of life if their cancer is treated than if they wait for disease progression. They have a very high chance of dying from another cause, and treatment of their prostate cancer could actually worsen comorbid (e.g. cardiac) disease and hasten death.
Radiation Therapy
External-beam radiation therapy: Radiation therapy also offers the potential for curative treatment of localized prostate cancer. It may be delivered in the form of external-beam radiation therapy (EBRT) or brachytherapy (i.e. the insertion of radioactive seeds into the prostate gland). EBRT techniques include 3-dimensional conformal radiation therapy (3D-CRT) and intensity-modulated radiation therapy (IMRT). Higher-dose-rate therapy using stereotactic guidance is being used despite lack of data on long-term survival or complication rates.
In general, after 2 years, the quality-of-life profile for IMRT and surgery are similar, although radiation therapy does pose a slightly higher risk of persistent fecal urgency and incontinence of gas. Proton-beam therapy is theoretically an excellent modality for EBRT, providing an ideal dose distribution.
Complications of EBRT include cystitis, proctitis, enteritis, impotence, urinary retention, and incontinence. Rates depend on the total dose and the technique used.
Brachytherapy: In 2011, the American Society for Radiation Oncology (ASTRO) and the American College of Radiology (ACR) issued a practice guideline for transperineal permanent brachytherapy of prostate cancer. These guidelines established standards for the safe and effective performance of brachytherapy for patients with organ-confined prostate cancer.(18)
Radiation therapy plus androgen ablation therapy: Androgen ablation has been shown to improve survival in men with localized disease who are treated with external radiation.
Radiation therapy versus surgery: The AHRQ found insufficient evidence to determine whether any type of radiation therapy results in fewer deaths or cancer recurrences than radical prostatectomy does in patients with clinically localized prostate cancer. The importance of dose escalation in disease control complicates the extraction of meaningful conclusions from current radiation therapy treatments (e.g. 3D-CRT, IMRT).
Brachytherapy has also been compared with surgery in the management of early stage disease. Direct comparisons (i.e. prospective, randomized trials) are not readily available, but preliminary data from most centers suggest that permanent prostate implants yield comparable local control and biochemical disease-free rates.
Non–Organ-Confined Disease
When imaging studies provide clear evidence of non–organ-confined disease (e.g. seminal vesicle or periprostatic involvement), the treatments offered may vary. Typically, some combination of modalities is involved.
Survival of men with locally advanced prostate cancer (T3-4N0M0) is improved by combining external radiation with androgen ablation for 6 months. If brachytherapy is used, it is often combined with EBRT and ADT, although studies demonstrating an improved outcome with combined radiation are also lacking. Because of the aggressive nature of these tumors, active surveillance is an option only in highly selected patients with life expectancies of less than 5 years.
Radical prostatectomy: Historically, radical prostatectomy for clinical stage T3 prostate cancer at initial presentation was not considered beneficial, because of the increased probability of incomplete resection of the cancer, the likelihood of micro-metastatic disease, and increased morbidity. For patients with T3a disease, however, current National Comprehensive Cancer Network guidelines recommend radical prostatectomy plus pelvic lymph node dissection as an option for initial therapy.
Adjuvant therapy: Radical prostatectomy followed by adjuvant radiation is also an option, but the rate of adverse effects with this approach is higher than with external radiation and hormone therapy. In addition, proof is lacking as to whether it offers comparable survival to radiation and hormone therapy.
Neoadjuvant hormone therapy has been used to clinically down-stage patients before surgery; however, all randomized studies to date have failed to show a significant benefit in either disease recurrence or survival.
Androgen ablation: Reports from the Memorial Sloan-Kettering Cancer Center suggest that long-term survival rates (i.e. ≥15 y) are essentially zero in the setting of synchronous nodal involvement at diagnosis. In this group of patients, hormone blockade with or without EBRT is used. Patients in whom non–organ-confined disease is suspected or confirmed but metastases are absent typically receive radiation therapy with hormone manipulation (LHRH agonist or antagonist treatment). The effect of chemotherapy in this setting has not been well studied.
Androgen ablation commonly begins several months before radiation is initiated and continues for several months or years afterward. However, the optimum sequencing of androgen blockade and radiation therapy remains unclear. So far, the best results have occurred with 3 years of therapy, but those studies were performed when lower doses of radiation were used without IMRT techniques. Additional studies would be needed using higher doses of radiation to determine whether the same benefit would occur.
ADT produces a range of adverse effects, but these symptoms usually diminish or disappear after the hormone therapy is discontinued. However, awareness and treatment of the various side effects of this therapy are important for a man’s quality of life and for reducing the morbidity of this therapy.
Management of Advanced and Metastatic Disease
A rise in PSA after radical prostatectomy to greater than 0.2 ng/mL or three consecutive PSA increases after radiation therapy are evidence of impending disease progression. Not all men who have a rising PSA will develop metastases, and for that reason not all such men require treatment. The risk of metastases and death depend on the patient’s Gleason score, the length of time between the nadir PSA and the onset of the PSA’s rise, and the PSA doubling time.
Patients who have PSA (biochemical) failure following radical prostatectomy and have no evidence of metastatic disease have the options of watchful waiting, radiation therapy, or hormone ablation as salvage therapy. Similarly, patients who have PSA failure following radiation therapy have the following options:
- Watchful waiting
- Brachytherapy
- Prostatectomy
- Cystoprostatectomy
- Cryotherapy
- Hormone ablation
The 2017 NCCN guideline(17) and the 2011 European Association of Urology (EAU) guideline(19) provide recommendations for treating patients with advanced prostate cancer in whom local therapy has failed.
Therapeutic options include the following:
- LHRH agonists – Available in 1-month, 3-month, 6-month, and once-yearly depots
- LHRH antagonist – Available in a 1-month depot
- Complete androgen blockade – LHRH agonist or antagonist with an oral antiandrogen
- Nonsteroidal antiandrogen monotherapy
- Bilateral orchiectomy
Investigational therapies
Investigational therapies for recurrent prostate cancer include high-intensity focused ultrasound (HIFU) and gene therapy. Other options include cellular immunotherapy, tumor vaccines, or vaccination with tumor- or prostate-specific proteins such as PSA.
Androgen Suppression in Advanced and Metastatic Disease
Androgen deprivation is considered the primary approach to the treatment of metastatic prostate cancer. However, ADT has been found to be palliative, not curative. Prior to the development of newer therapies, overall survival for patients with metastatic prostate cancer ranged from 24-36 months. In recent years, however, several new therapies have been approved that prolong survival in men whose disease progresses on ADT.
Adverse effects of androgen suppression: Surgical and medical castration lead to a number of side effects, including the following, that can have a significant impact on a man’s quality of life:
- Anemia
- Breast enlargement
- Cognitive impairment
- Decreased libido
- Decreased muscle mass
- Erectile dysfunction
- Fatigue
- Fractures
- Gastrointestinal tract disturbances
- Gynecomastia
- Hot flashes
- Osteoporosis
- Metabolic syndrome
- Pulmonary edema
- Psychological changes
- Weight gain
In addition, in men with prostate cancer receiving ADT, lean body mass decreases significantly after 12-36 months of treatment.
Surgical Management of Metastatic Disease
There is no evidence that radical prostatectomy conveys any benefits to patients with documented metastatic disease. However, transurethral resection is sometimes needed in men who develop obstruction secondary to local tumor growth.
Bilateral orchiectomy can be used to produce androgen deprivation in patients with widely advanced and metastatic prostate cancer. Since the introduction of LHRH agonist and antagonist therapies, surgical intervention has been practiced less often. An indication for immediate bilateral orchiectomy is spinal cord compression, because it avoids the potential flare response that can occur during the first 3 weeks of treatment with an LHRH agonist.
Management of Castrate-Resistant Prostate Cancer
Eventually, almost all patients with metastatic disease become resistant to androgen ablation. In patients with castrate serum testosterone levels (less than 50 ng/dL), castrate-resistant prostate cancer is defined as 2-3 consecutive rises in PSA levels obtained at intervals of greater than 2 weeks and/or documented disease progression based on findings from computed tomography (CT) scan and/or bone scan, bone pain, or obstructive voiding symptoms.
Rarely, a rise in PSA may reflect failure of LHRH treatment to control testosterone secretion, rather than the development of castrate-resistant disease. Therefore, the testosterone level should be measured when the PSA rises. If the serum testosterone level exceeds castrate levels, changing the antiandrogen therapy may drop the PSA and delay the need for other therapy.
Prior to the development of the most recent therapies, the median time to symptomatic progression after a rise in the PSA level of more than 4 ng/mL was approximately 6-8 months, with a median time to death of 12-18 months. Since then, however, the latter figure has increased.
Nonchemotherapy options that provide palliation and improve quality of life include the following:
- Megestrol
- Nonsteroidal antiandrogens
- Corticosteroids
- Ketoconazole
- Radiation therapy
- Bisphosphonates
- Suramin
- Estrogen
None of these have been shown to improve survival, although few of them have been properly assessed for that endpoint.
Docetaxel: Therapeutic options for patients with castrate-resistant prostate cancer have changed significantly in the past 7 years, beginning with the approval of docetaxel chemotherapy.
Other therapies now approved for androgen-independent prostate cancer include the following:
- Sipuleucel-T
- Abiraterone acetate
- Enzalutamide
- Cabazitaxel
- Mitoxantrone
- Apalutamide
Sipuleucel-T: Sipuleucel-T is a therapeutic vaccine that was approved by the FDA in April 2010 for asymptomatic or minimally symptomatic prostate cancer with metastases resistant to standard hormone treatment. The 2015 NCCN guideline supports its use in men with good performance status, a life expectancy of more than 6 months, no hepatic metastases, and no or minimal symptoms.(20)
Abiraterone acetate: Abiraterone acetate, an inhibitor of androgen biosynthesis, was approved by the FDA in April 2011 for use in combination with prednisone for the treatment of patients with metastatic, castrate-resistant prostate cancer who have received prior chemotherapy containing docetaxel. Abiraterone blocks CYP17A1, an enzyme that is important in the synthesis of testosterone by the adrenal gland and by prostate cancer cells. This results in blocking all testosterone production.
Enzalutamide: Enzalutamide acts by inhibiting the binding of androgens to the androgen receptor and inhibits translocation of the androgen receptor into the nucleus. In July 2018, the FDA approved an expanded indication for enzalutamide in CRPC to include patients with nonmetastatic disease.
Cabazitaxel: Cabazitaxel is another taxane that acts as a microtubular inhibitor. In a study of 755 men with metastatic, castrate-resistant prostate cancer that had progressed despite docetaxel treatment, the median overall survival period was 15.1 months in patients receiving cabazitaxel, versus 12.7 months in those receiving mitoxantrone. Median progression-free survival was also longer with cabazitaxel. Both groups also received prednisone. The most common clinically significant grade 3 or higher adverse events with cabazitaxel were neutropenia and diarrhea. Febrile neutropenia was also more common with cabazitaxel.(21)
Apalutamide: Apalutamide, an androgen receptor inhibitor, was approved by the FDA in February 2018 for treatment of nonmetastatic, castration-resistant prostate cancer (NM-CRPC).
Bone Protection in Metastatic Disease
Use of a bone-protective therapy is an important aspect of managing men with metastatic disease. Two agents are now approved for this indication: zoledronic acid, a bisphosphonate, and denosumab, an antibody that inhibits osteoclastic activity in bone. Both drugs delay the risk of skeletally related events by relieving bone pain, preventing fractures, decreasing the need for surgery and radiation to the bones, and lowering the risk of spinal cord compression.
Vitamin D and calcium should be taken as supplements with this therapy. In addition, patients should be monitored regularly for hypocalcemia.
GOALS OF THERAPY
The desired goals in early stage prostate cancer is to minimize morbidity and mortality caused by prostate cancer while minimizing toxicity associated with prostate cancer treatments. Unfortunately, the most appropriate therapy of early stage prostate cancer is unknown. Early stage disease may be treated with surgery, radiation, or watchful waiting.
GUIDELINES
To view, “European Association of Urology/European Society for Radiotherapy and Oncology/International Society of Geriatric Oncology (EAU/ESTRO/SIOG) guidelines on Prostate Cancer: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer”, please click on below link:
https://uroweb.org/wp-content/uploads/Cornford-P-et-al.-Eur-Urol-2017-714630.-EAU-ESTRO-SIOG-Guidelines-on-Prostate-Cancer.-Part-II.-Treatment-of-relapsing.-Prt-Vr..pdf
To view, “American College of Physicians (ACP) guidelines on Prostate Cancer Screening”, please click on below link:
https://www.acponline.org/acp-newsroom/american-college-of-physicians-releases-new-prostate-cancer-screening-guidance-statement
To view, “U.S. Preventive Services Task Force (USPSTF) guidelines on Prostate Cancer Screening”, please click on below link:
https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening1?ds=1&s=prostate
To view, “National Comprehensive Cancer Network (NCCN) guidelines on Prostate Cancer”, please click on below link:
https://www.nccn.org/patients/guidelines/prostate/files/assets/common/downloads/files/prostate.pdf
To view, “American Cancer Society (ACS) guidelines on Prostate Cancer”, please click on below link:
https://www.cancer.org/cancer/prostate-cancer.html
To view, “American Urological Association (AUA) guidelines on Early Detection of Prostate Cancer”, please click on below link:
https://www.auanet.org/guidelines/prostate-cancer-early-detection-(2013-reviewed-for-currency-2018)
To view, “Cancer of the prostate: European Society for Medical Oncology (ESMO) Clinical Practice Guidelines for diagnosis, treatment and follow-up”, please click on below link:
https://www.esmo.org/Guidelines/Genitourinary-Cancers/Cancer-of-the-Prostate/eUpdate-Treatment-Recommendation
CONSULTATION AND LONG TERM MONITORING
Follow-up is not standardized; however, practitioners use general guidelines that are derived mainly from publications report outcomes on various methods of treatment.
Consultation with a radiation oncologist should be obtained for palliative radiation therapy for bone metastases, for locally extensive tumors, and, on an emergent basis, for spinal cord compression. Consultations with a neurosurgeon for spinal cord compression and an orthopedic surgeon for pathologic fractures are appropriate. Consultation with a medical oncologist may also be considered for chemotherapy when a patient with metastatic disease begins to show disease progression on hormone therapy.
PRECAUTIONS
Lifestyle measures
Diets associated with a reduced risk of prostate cancer in epidemiologic studies are composed mainly of vegetables, fruits, grains, and fish. Tomatoes (because of their lycopene content), broccoli, green tea, and soy have all been hypothesized to be beneficial.
Increased risk has been shown with high-fat diets, excessive intake of estrogens and phytoestrogens, and the consumption of burned or charred foods. Obesity appears to be the diet-related factor most strongly associated with prostate cancer, so overall energy intake is important.
Because a high-fat diet is linked with a higher incidence of prostate cancer, a low-fat diet may be beneficial for men at high risk of developing prostate cancer (i.e. those with a positive family history, African Americans) and for patients undergoing treatment for advanced prostate cancer. However, no prospective studies have proved that dietary modification provides a benefit.
Physical activity
Physical activity appears to lower prostate cancer risk.
It is common for men to notice a decline in fitness level and muscle strength after treatment. Becoming active again or introducing a fitness routine is an important step to regaining health. Regular physical activity is shown to have a number of health benefits, including:
- Improving cardiovascular health.
- Reducing anxiety and depression.
- Maintaining a healthy weight.
- Improving muscle strength.
Food safety precautions for people with prostate cancer
Infection is a concern for people living with cancer, especially during times when the immune system is weak. Handling and storing food safely is an important precaution to reduce exposure to unsafe germs and bacteria.
Food safety tips:
- Wash hands before eating or preparing foods.
- Wash vegetables and fruits.
- Standard guidelines recommend keeping foods colder than 41°F and hotter than 140°F.
- Keep raw meats and meat juices away from other foods.
REFERENCES
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69:7.
- American Cancer Society. Cancer Facts & Figures 2018. Available at https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf. Accessed: February 16, 2018.
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics. CA: Cancer J Clin 2015; 65: 5-29.
- Bhurgri Y, Kayani N, Pervez S, Ahmed R, Tahir I, Afif M, et al. Incidence and trends of prostate cancer in Karachi South, 1995- 2002. Asian Pac J Cancer Prev 2009; 10: 45-8.
- Pakzad R, Mohammadian-Hafshejani A, Ghoncheh M, Pakzad I, Salehiniya H. The incidence and mortality of prostate cancer and its relationship with development in Asia. Prostate international. 2015 Dec 1;3(4):135-40.
- Gerald W Chodak. Prostate Cancer. Medscape. Available from: https://emedicine.medscape.com/article/1967731-overview#a4 (Accessed on 2019 Jan 21)
- Rosenberg MT, Froehner M, Albala D, Miner MM. Biology and natural history of prostate cancer and the role of chemoprevention. International journal of clinical practice. 2010 Dec;64(13):1746-53.
- National Comprehensive Cancer Network. Prostate Cancer Early Detection. National Comprehensive Cancer Network. Available at https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf. Version 2.2017 — September 15, 2017; Accessed: February 16, 2018
- American Cancer Society Recommendations for Prostate Cancer Early Detection. American Cancer Society. Available at http://www.cancer.org/Cancer/ProstateCancer/MoreInformation/ProstateCancerEarlyDetection/prostate-cancer-early-detection-acs-recommendations. April 14, 2016; Accessed: February 16, 2018.
- Bul M, van Leeuwen PJ, Zhu X, Schröder FH, Roobol MJ. Prostate cancer incidence and disease-specific survival of men with initial prostate-specific antigen less than 3.0 ng/ml who are participating in ERSPC Rotterdam. Eur Urol. 2011 Apr. 59(4):498-505
- Bul M, van Leeuwen PJ, Zhu X, Schröder FH, Roobol MJ. Prostate cancer incidence and disease-specific survival of men with initial prostate-specific antigen less than 3.0 ng/ml who are participating in ERSPC Rotterdam. Eur Urol. 2011 Apr. 59(4):498-505
- National Cancer Institute. Prostate Cancer Screening (PDQ®). Available at http://www.cancer.gov/cancertopics/pdq/screening/prostate/HealthProfessional/allpages. February 9, 2018; Accessed: February 16, 2018
- American College of Radiology. ACR Appropriateness Criteria® prostate cancer—pretreatment detection, surveillance, and staging. Naitonal Guideline Clearinghouse. Available at https://guideline.gov/summaries/summary/50643. 2016; Accessed: February 16, 2018.
- Buyyounouski MK, Choyke PL, McKenney JK, Sartor O, Sandler HM, Amin MB, et al. Prostate cancer – major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017 May 6. 67 (3):245-253
- Thompson I, Thrasher JB, Aus G, Burnett AL, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007 Jun. 177(6):2106-31
- Boorjian SA, Thompson RH, Siddiqui S, Bagniewski S, Bergstralh EJ, Karnes RJ, et al. Long-term outcome after radical prostatectomy for patients with lymph node positive prostate cancer in the prostate specific antigen era. J Urol. 2007 Sep. 178(3 Pt 1):864-70; discussion 870-1
- Prostate Cancer. National Comprehensive Cancer Network. Available at http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Version 1.2018 — February 14, 2018; Accessed: February 16, 2018
- Rosenthal SA, Bittner NH, Beyer DC, Demanes DJ, et al. American Society for Radiation Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys. 2011 Feb 1. 79(2):335-41.
- Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011 Apr. 59(4):572-83
- Schumacher MC, Burkhard FC, Thalmann GN, Fleischmann A, Studer UE. Good outcome for patients with few lymph node metastases after radical retropubic prostatectomy. Eur Urol. 2008 Aug. 54(2):344-52
- de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP,et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010 Oct 2. 376(9747):1147-54
