EPIDEMIOLOGY
It is estimated that approximately two billion people worldwide have evidence of past or present infection with hepatitis B virus (HBV), and 248 million individuals are chronic carriers (i.e. positive for hepatitis B surface antigen [HBsAg]).(1,2) The overall prevalence of HBsAg is reported to be 3.6 percent; however, it varies depending upon the geographic area. The prevalence of chronic HBV ranges from <2 percent in low-prevalence areas (e.g. United States, Canada, Western Europe) to 2 to 7 percent in intermediate-prevalence areas (e.g. Mediterranean countries, Japan, Central Asia, Middle East, and parts of South America) to ≥8 percent in high-prevalence areas (e.g. Western Africa, South Sudan).(1-3)
Pakistan is highly endemic with HBV(4) with nine million people infected with HBV(5) and its infection rate is on a steady rise.(6)
PATHOPHYSIOLOGY(7)
Hepatitis B virus (HBV) is a hepadnavirus, with the virion consisting of a 42-nm spherical, double-shelled particle composed of small spheres and rods and with an average width of 22 nm.(8-12) It is an exceedingly resistant virus, capable of withstanding extreme temperatures and humidity. HBV can survive when stored for 15 years at –20°C, for 24 months at –80°C, for 6 months at room temperature, and for 7 days at 44°C.
Viral genome
The viral genome of hepatitis B consists of a partially double-stranded, circular DNA molecule of 3.2 kilobase (kb) pairs that encodes the following 4 overlapping open reading frames:
- S (the surface, or envelope, gene): Encodes the pre-S1, pre-S2, and S proteins
- C (the core gene): Encodes the core nucleocapsid protein and the e antigen; an upstream region for the S (pre-S) and C (pre-C) genes has been found
- X (the X gene): Encodes the X protein
- P (the polymerase gene): Encodes a large protein promoting priming ribonucleic acid (RNA) ̶ dependent and DNA-dependent DNA polymerase and ribonuclease H (RNase H) activities
Surface gene
The S gene encodes the viral envelope. There are 5 mainly antigenic determinants: (1) a, common to all hepatitis B surface antigen (HBsAg), and (2-5) d, y, w, and r, which are epidemiologically important and identify serotypes.
Core gene
The core antigen, HBcAg, is the protein that encloses the viral DNA. It can also be expressed on the surface of the hepatocytes, initiating a cellular immune response. The e antigen, HBeAg, which is also produced from the region in and near the core gene, is a marker of active viral replication. It serves as an immune decoy and directly manipulates the immune system; it is thus involved in maintaining viral persistence. HBeAg can be detected in patients with circulating serum HBV DNA who have “wild type” infection. As the virus evolves over time under immune pressure, core promotor and precore mutations emerge, and HBeAg levels fall until the level is not measurable by standard assays.
Individuals who are infected with the wild type virus often have mixed infections, with core and precore mutants in up to 50% of individuals. They often relapse with HBeAg-negative disease after treatment.
X gene
The role of the X gene is to encode proteins that act as transcriptional transactivators that aid viral replication. Evidence strongly suggests that these transactivators may be involved in carcinogenesis.
Antibody production
The production of antibodies against HBsAg (anti-HBs) confers protective immunity after vaccination and can be detected in patients who have recovered from HBV infection or in those who have been vaccinated. Antibody to HBcAg (anti-HBc) is detected in almost every patient with previous exposure to HBV and indicates that there is a minute level of persistent virus, as demonstrated by the risk of reactivation in individuals who undergo immune suppression regardless of their anti-HBs status.
The immunoglobulin M (IgM) subtype of anti-HBc is indicative of acute infection or reactivation, whereas the IgG subtype is indicative of chronic infection. The activity of the disease cannot be understood using this marker alone. Antibody to HBeAg may be suggestive of a nonreplicative state if there is undetectable HBV DNA or the emergence of the core / precore variants and of chronic HBV HBeAg-negative disease.
Variants of HBV
With the newest polymerase chain reaction (PCR) assay techniques, scientists are able to identify variations in the HBV genome (variants) as far back as 1995, even in patients who are positive for HBeAg. Mutations of various nucleotides such as the 1896, 1764, and 1768 (precore/core region) processing the production of the HBeAg have been identified (HBeAg-negative strain).(13)
Immune response
The pathogenesis and clinical manifestations of hepatitis B infection are due to the interaction of the virus and the host immune system. The immune system attacks HBV and causes liver injury, the result of an immunologic reaction when activated CD4+ and CD8+ lymphocytes recognize various HBV-derived peptides on the surface of the hepatocytes. Impaired immune reactions (e.g. cytokine release, antibody production) or a relatively tolerant immune status result in chronic hepatitis. In particular, a restricted T-cell–mediated lymphocytic response occurs against the HBV-infected hepatocytes.(14,15)
The final state of HBV disease is cirrhosis. With or without cirrhosis, however, patients with HBV infection are likely to develop hepatocellular carcinoma (HCC).(16-18)
Genotypes and disease progression
Ten different genotypes (A through J), representing a divergence of the viral DNA of about 8%, have been identified.(19) The prevalence of the genotypes varies in different countries. The progression of the disease seems to be more accelerated and the response to treatment with antiviral agents is less favorable for patients infected by genotype C, compared with those infected by genotype B. However, much of this can be explained by the presence of core and precore mutations found in multivariate analysis.(20,21)
TRANSMISSION
Hepatitis B virus (HBV) is transmitted from patients who are infected to those who are not immune (i.e. hepatitis B surface antibody [anti-HBs]-negative). Hepatitis B vaccination has significantly reduced the risk of transmission worldwide.
Following are the different modes of transmission.
Mother-to-child transmission:
The infection rate of infants born to hepatitis B surface antigen (HBsAg)-positive mothers is as high as 90 percent among infants who do not receive hepatitis B immune globulin and hepatitis B vaccination at birth.(24) Mother-to-child transmission may occur in utero, at the time of birth, or after birth. However, most infections occur at the time of birth.
Breastfeeding:
Breastfeeding does not appear to increase the risk of transmission.
Paternal transmission:
Transmission of HBV from fathers to their infants is possible based upon genotypic and phylogenetic analysis. In a study conducted in Taiwan, the HBV infection rate was 65 percent among neonates born to HBsAg-negative mothers and HBsAg-positive fathers.(25) Most of these transmissions are believed to result from close contact of the unprotected infants with the infected blood and body fluids of the fathers. Although some studies have detected HBV in sperm, there is no clinical evidence to support that infected sperm result in transmission of HBV infection to the fetus.(26-29)
Transfusion:
The World Health Organization suggests screening with both HBsAg and hepatitis B core antibody (anti-HBc).(30) The risk of HBV transmission through blood transfusions was significantly reduced after the introduction of serologic screening of donors for HBsAg.(31) The risk was further reduced by screening for anti-HBc in addition to HBsAg. Anti-HBc can be detected during the widow phase when HBsAg is not present.
Sexual transmission:
Sexual transmission remains a common source of HBV transmission.(32-34) Unvaccinated men who have sex with men and heterosexual persons who have multiple sex partners or contact with sex workers are at particularly high risk.(35)
Percutaneous inoculation:
Percutaneous transmission usually happens among injection drug users (IDU) who share syringes and needles. The risk of HBV transmission increases with number of years of drug use, frequency of injection, and sharing of drug preparation equipment.(36-38) In addition to drug use, certain practices such as acupuncture, tattooing, and body piercing have also been associated with transmission of HBV through the use of equipment that is contaminated with HBV-infected blood.(34)
Nosocomial infection:
HBV can be transmitted in the health care setting.(39,40) Transmission generally occurs from patient to patient or from patient to health care providers (HCP) via contaminated instruments or an accidental needle stick. The number of HBV infections among HCP has declined significantly, due in large part to efforts aimed at immunizing all HCP against HBV and using postexposure prophylaxis for nonimmune persons (i.e. those who are unvaccinated or vaccine nonresponders). Among individuals who are not immune to HBV, the risk of transmission depends upon the HBsAg, HBeAg, and HBV DNA status of the source.
Although uncommon, transmission can also occur from an infected HCP to a nonimmune patient. Transmission usually results from unsafe injection practices, which often could have been avoided with standard precautions and appropriate aseptic techniques.(41)
Transplant recipients:
HBV infection can be transmitted from HBsAg-positive donors to HBsAg-negative recipients, with severe clinical consequences when the recipient is nonimmune (i.e. anti-HBs-negative).
Other modes of transmission:
Adults and children may acquire HBV infection via blood exposure to minor breaks in the skin or mucous membranes. In addition, transmission can occur via exposure to household articles that have been contaminated with blood, such as toothbrushes, razors, and toys, since HBV can survive outside the human body for a prolonged period.(42) Although HBV DNA has been detected in various bodily secretions of hepatitis B carriers, there is no firm evidence of HBV transmission via body fluids other than blood or semen.
NATURAL HISTORY(43)
Phases of Chronic HBV Infection:
Chronic HBV infection generally consists of two phases: an early replicative phase with active liver disease (immune-active chronic HBV), and a later phase with low replication and remission of liver disease (inactive chronic HBV) (figure 1).(44,45) In patients with a perinatally acquired HBV infection, there is an additional immune tolerance phase in which virus replication is not accompanied by active liver disease (figure 2).(46) In some patients, reactivation of HBV replication occurs after a varying period of quiescence.
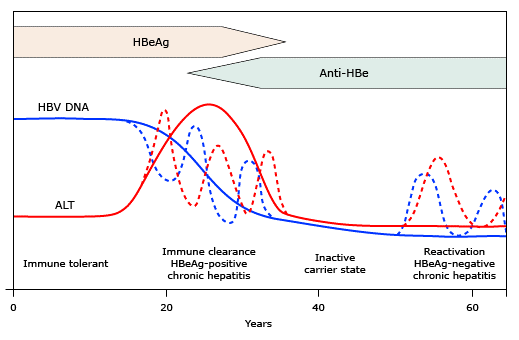
Figure 1: The course of chronic HBV infection is considered to consist of four phases: immune tolerance, immune clearance (HBeAg-positive chronic hepatitis), inactive carrier, and reactivation (HBeAg-negative chronic hepatitis), although not all patients go through every phase.
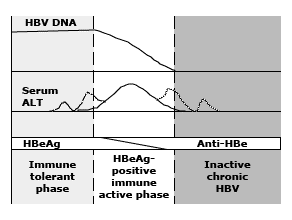
Figure 2: Schematic representation of the natural history of perinatally acquired chronic hepatitis B virus (HBV) infection.
SIGN AND SYMPTOMS(43)
Immune tolerance: In patients with a perinatally acquired HBV infection, the initial phase is characterized by high levels of HBV replication—the presence of hepatitis B e antigen (HBeAg) and high levels of HBV DNA in serum—but no evidence of active liver disease as manifested by lack of symptoms, normal serum ALT concentrations, and minimal changes on liver biopsy (figure 2).(47,48)
The lack of liver disease despite high levels of HBV replication has typically been attributed to immune tolerance to HBV.(49) Immune tolerance is also believed to be the major reason for the poor response to interferon therapy in HBeAg positive Asian patients who have normal serum ALT concentrations. The immune tolerance phase usually lasts 10 to 30 years, during which there is a very low rate of spontaneous HBeAg clearance.(50,51)
Immune-active, HBeAg-positive: The transition from the immune tolerance to the immune-active or clearance phase occurs during the second and third decades in patients with perinatally acquired HBV infection. During this phase, spontaneous HBeAg clearance increases to an annual rate of 10 to 20 percent.(50,51)
HBeAg seroconversion is frequently, but not always, accompanied by biochemical exacerbations (abrupt increases in serum ALT) (figure 2). Exacerbations are believed to be due to a sudden increase in immune-mediated lysis of infected hepatocytes. They are often preceded by an increase in serum HBV DNA(52) and a shift of HBcAg (hepatitis B core antigen) from nuclear to cytoplasmic sites within hepatocytes,(53) suggesting that immune clearance may be triggered by an increase in viral load or a change in the presentation of viral antigens.
Inactive chronic HBV: Patients in the low or nonreplicating phase/inactive carrier state are HBeAg negative and anti-HBe positive. In some patients, HBV DNA is undetectable in serum by polymerase chain reaction assays, and liver disease is in remission as evidenced by normal serum ALT concentrations and the resolution of necroinflammation in liver biopsies.
HBeAg-negative patients with a persistently normal serum ALT can still have significant histologic inflammation and/or fibrosis,(54,55) although a meta-analysis found that significant liver disease was rare in those with a persistently normal ALT and an HBV DNA level ≤20,000 international units/mL.(56)
Because of the fluctuating nature of chronic HBV infection, patients should not be categorized as inactive carriers unless there are at least three ALT levels and two to three HBV DNA levels over a 12-month period of observation.
Immune-active, HBeAg-negative: Some patients continue to have moderate levels of HBV replication and active liver disease (elevated serum ALT and chronic inflammation on liver biopsies), but remain HBeAg negative.(57,58) Such patients are said to have HBeAg-negative chronic hepatitis. They have a residual wild-type virus or HBV variants that cannot produce HBeAg due to precore or core promoter genetic variations.59-62)
Patients with HBeAg-negative immune-active chronic hepatitis B are older and have more advanced liver disease. They also tend to have fluctuations in HBV DNA and ALT levels.
Resolution of chronic HBV infection: Some patients with chronic HBV infection become HBsAg negative.
In most reports, patients who cleared HBsAg appeared to have a good prognosis.(63-68) In the absence of other causes of liver injury, progression to cirrhosis and hepatic decompensation after HBsAg clearance is rare. However, the risk of hepatocellular carcinoma remains, and surveillance should continue in those who have HCV or hepatitis D virus (HDV) coinfection, cirrhosis, or are older than 50 years at the time of HBsAg clearance.
Acute hepatitis
Approximately 70 percent of patients with acute hepatitis B virus (HBV) infection have subclinical or anicteric hepatitis, while 30 percent develop icteric hepatitis. The disease may be more severe in patients coinfected with other hepatitis viruses or with underlying liver disease.(69)
The incubation period lasts one to four months. A serum sickness-like syndrome may develop during the prodromal period, followed by constitutional symptoms, anorexia, nausea, jaundice, and right upper quadrant discomfort. The symptoms and jaundice generally disappear after one to three months, but some patients have prolonged fatigue even after normalization of serum aminotransferase concentrations.
Laboratory testing during the acute phase reveals elevations in the concentration of alanine and aspartate aminotransferase levels (ALT and AST); values up to 1000 to 2000 units/L are typically seen during the acute phase with ALT being higher than AST. The serum bilirubin concentration may be normal in patients with anicteric hepatitis. The prothrombin time is the best indicator of prognosis. In patients who recover, the normalization of serum aminotransferases usually occurs within one to four months. A persistent elevation of serum ALT for more than six months indicates a progression to chronic hepatitis.
Chronic Hepatitis
Many patients with chronic HBV are asymptomatic (unless they have decompensated cirrhosis or have extrahepatic manifestations), while others have nonspecific symptoms such as fatigue. Some patients experience exacerbations of the infection which may be asymptomatic, mimic acute hepatitis, or manifest as hepatic failure.
Physical examination may be normal, or there may be stigmata of chronic liver disease. Jaundice, splenomegaly, ascites, peripheral edema, and encephalopathy may be present in patients with decompensated cirrhosis. Laboratory tests may be normal, but most patients have a mild to moderate elevation in serum AST and ALT. During exacerbations, the serum ALT concentration may be as high as 50 times the upper limit of normal, and alpha-fetoprotein (AFP) concentrations as high as 1000 ng/mL may be seen.(70) A progression to cirrhosis is suspected when there is evidence of hypersplenism (decreased white blood cell and platelet counts) or impaired hepatic synthetic function (hypoalbuminemia, prolonged prothrombin time, hyperbilirubinemia).
Extrahepatic manifestations: Extrahepatic manifestations, which are thought to be mediated by circulating immune complexes, occur in 10 to 20 percent of patients with chronic HBV infection. The two major extrahepatic complications of chronic HBV are polyarteritis nodosa and glomerular disease.
SCREENING
Screening of the following groups of asymptomatic individuals for HBV regardless of their vaccination history should be performed:
- Persons born in countries with HBV prevalence ≥2 percent
- Pregnant women
- Those requiring immunosuppressive therapy
- Blood, plasma, organ, tissue, or semen donors
- Infants born to HBV-infected mothers
In addition, screening should be performed for asymptomatic individuals who are at high risk for having HBV infection if they were not vaccinated or were vaccinated but did not have screening prior to vaccination. These individuals include:
- Persons born in the high prevalence countries
- Persons with HIV or hepatitis C virus
- Persons who have ever injected drugs
- Men who have sex with men
- Individuals with multiple sexual partners and/or a history of sexually transmitted diseases
- Patients with end-stage renal disease (including those undergoing dialysis)
- Household and sexual contacts of HBV-infected persons
- Inmates of correctional facilities
- Individuals with chronic liver disease (e.g. cirrhosis, fatty liver disease, autoimmune liver disease, ALT or AST greater than twice the upper limit of normal)
The following groups may not have any of the above risk factors, but they require vaccination because of occupational, recreational, or comorbid conditions that put them at risk for HBV. For such patients, the goal of screening is to avoid unnecessary vaccination. However, if screening is not possible due to time constraints (e.g. a traveler) or logistics (e.g. desire to initiate vaccination as soon as possible or to minimize steps and time required to initiate vaccination), vaccination should be administered regardless.
- International travelers to regions with high (≥8 percent) or intermediate (2 to 7 percent) prevalence of HBV infection.
- Residents and staff of facilities for developmentally disabled persons
- Health care and public safety workers with potential exposure to blood or blood-contaminated body fluids
Adults age 19 through 59 years with diabetes mellitus.
DIAGNOSTIC TESTS(71)
For patients with clinical signs and symptoms of hepatitis B virus (HBV) infection, tests to order needs to be determine based upon the clinical presentation (e.g. acute versus chronic infection). This is discussed in detail below.
For screening of asymptomatic persons:
- Hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (anti-HBs) test is recommended.(72)
- Testing for IgG hepatitis B core antibody (anti-HBc) is also performed in the following groups:
- Patients with HIV.
- Those with hepatitis C virus (HCV) who are going to undergo treatment.
- Patients who require immunosuppressive therapy.
- Blood and organ donors.
Testing for anti-HBc allows one to differentiate immunity from HBV vaccination versus recovery from past HBV infection and helps identify those with occult infection. This is important because patients with prior or occult HBV may be at risk for reactivation in certain settings. In addition, patients with isolated anti-HBc can transmit HBV to others via blood or organ donation on rare occasion.
Types of tests
Serologic markers
Infection with HBV is associated with characteristic changes in the serum levels of hepatitis B antigens and antibodies. These markers are used to define different clinical states.
Hepatitis B surface antigen and antibody: Hepatitis B surface antigen (HBsAg) is the serologic hallmark of HBV infection. It can be detected using an enzyme immunoassay (EIA).
HBsAg appears in serum 1 to 10 weeks after an acute exposure to HBV, prior to the onset of hepatitic symptoms or elevation of serum alanine aminotransferase (ALT). In patients who subsequently recover, HBsAg usually becomes undetectable after four to six months. Persistence of HBsAg for more than six months implies chronic infection.
The disappearance of HBsAg is followed by the appearance of hepatitis B surface antibody (anti-HBs). In most patients, anti-HBs persists for life, thereby conferring long-term immunity. In some patients, however, anti-HBs may not be detectable until after a window period of several weeks to months, during which neither HBsAg nor anti-HBs can be detected (figure 3). At this time, the serologic diagnosis may be made by the detection of IgM antibodies against hepatitis B core antigen (IgM anti-HBc).
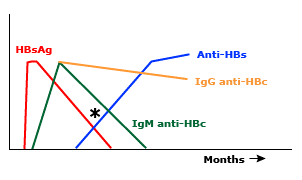
Figure 3: Schematic representation of the serologic findings during the window period of acute hepatitis B virus infection. The disappearance of HBsAg (hepatitis B surface antigen) is followed by the appearance of anti-HBs. In some patients, however, anti-HBs may not be detectable until after a window period of several weeks to months. At this time, neither HBsAg nor anti-HBs can be detected, the serologic diagnosis may be made by the detection of IgM antibodies against hepatitis B core antigen (IgM anti-HBc).
Hepatitis B core antigen and antibody: Hepatitis B core antigen (HBcAg) is an intracellular antigen that is expressed in infected hepatocytes. It is not detectable in serum. Antibody to hepatitis B core antigen (anti-HBc) can be detected throughout the course of HBV infection.
During acute infection, anti-HBc is predominantly of IgM class (Figure 4). IgM anti-HBc is the sole marker of HBV infection during the window period between the disappearance of HBsAg and the appearance of anti-HBs (Figure 3). The detection of IgM anti-HBc is usually regarded as an indication of acute HBV infection.
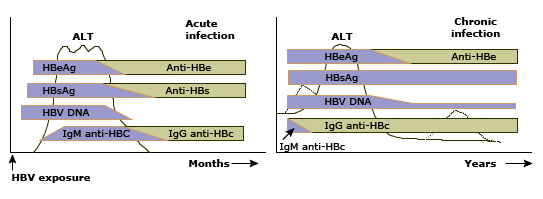
Figure 4: Schematic representation of the serologic responses to acute and chronic hepatitis B virus (HBV) infection in relation to the serum alanine aminotransferase (ALT) concentration. Left panel: Acute infection is characterized initially by the presence of HBeAg (hepatitis B e antigen), HBsAg (hepatitis B surface antigen), and HBV DNA beginning in the preclinical phase. IgM anti-HBc (hepatitis B core antigen) appears early in the clinical phase; the combination of this antibody and HBsAg makes the diagnosis of acute infection. Recovery is accompanied by normalization of the serum ALT, the disappearance of HBV DNA, HBeAg to anti-HBe seroconversion, and subsequently HBsAg to anti-HBs seroconversion and switch from IgM to IgG anti-HBc. Thus, previous HBV infection is characterized by anti-HBs and IgG anti-HBc. Right panel: Chronic infection is characterized by persistence of HBeAg (for a variable period), HBsAg, and HBV DNA in the circulation; anti-HBs is not seen (in approximately 20 percent of patients a non-neutralizing form of anti-HBs can be detected). Persistence of HBsAg for more than six months after acute infection is considered indicative of chronic infection.
However, IgM anti-HBc may remain detectable up to two years after the acute infection. Furthermore, the titer of IgM anti-HBc may increase to detectable levels during exacerbations of chronic hepatitis B.(73) This can present a diagnostic problem: incorrectly suggesting acute hepatitis B, particularly in endemic areas in which many HBsAg-positive patients presenting with acute hepatitis actually have exacerbations of chronic hepatitis B.(74,75) Other common causes of acute exacerbation of chronic hepatitis B are superinfection with hepatitis D virus (delta virus) or hepatitis C virus.(74,75)
IgG anti-HBc persists along with anti-HBs in patients who recover from acute hepatitis B (figure 4). It also persists in association with HBsAg in those who progress to chronic HBV infection.
Isolated anti-HBc: Isolated detection of anti-HBc can occur in several settings:
- During the window period of acute hepatitis B when the anti-HBc is predominantly IgM class.
- When anti-HBs has fallen to undetectable levels many years after recovery from acute hepatitis B.
- When the HBsAg titer has fallen below the cutoff level for detection in those with chronic hepatitis B.
- In the setting of HBsAg mutations where testing leads to false-negative HBsAg results. This occurs when monoclonal instead of polyclonal hepatitis B surface antibodies are used in enzyme immunoassays for capture and/or detection of HBsAg.(76,77)
- In some cases, isolated anti-HBc may be due to a false-positive test result. This is most likely seen in patients who are at low risk for disease.
Hepatitis B e antigen and antibody: Hepatitis B e antigen (HBeAg) is a secretory protein that is processed from the precore protein. It is generally considered to be a marker of HBV replication and infectivity. The presence of HBeAg is usually associated with high levels of HBV DNA in serum and higher rates of transmission of HBV infection from carrier mothers to their babies.(78-80) and from patients to health care workers.(81)
HBeAg to anti-HBe seroconversion occurs early in patients with acute infection, prior to HBsAg to anti-HBs seroconversion (figure 4). However, HBeAg seroconversion may be delayed for years to decades in patients with chronic HBV infection. In such patients, the presence of HBeAg is usually associated with the detection of high levels of HBV DNA in serum and active liver disease. However, HBeAg-positive patients with perinatally acquired HBV infection may have normal serum ALT concentrations and minimal inflammation in the liver.(47,48)
Serum HBV DNA assays: Qualitative and quantitative tests for HBV DNA in serum have been developed to assess HBV replication. Recovery from acute hepatitis B is usually accompanied by the disappearance of HBV DNA in serum as determined by hybridization or branched DNA (bDNA) assays. However, HBV DNA may remain detectable in serum for many years if tested by PCR assays.(82) This observation suggests that the virus persists after “recovery” but is controlled by the immune system.
Similar findings have been noted in patients with chronic HBV infection. Spontaneous or treatment-induced HBeAg seroconversion is usually accompanied by the disappearance of HBV DNA from serum by hybridization methods, but PCR assays usually remain positive except in patients with HBsAg seroconversion.(83)
HBV DNA levels are also detectable in patients with HBeAg-negative chronic hepatitis, although levels are generally lower than in patients with HBeAg-positive chronic hepatitis. A serum HBV DNA level of >2,000 international units/mL has been proposed as a cutoff level to differentiate patients with HBeAg-negative chronic hepatitis from those in an inactive carrier state (i.e. HBeAg-negative, persistently normal ALT).(84,85) Because of the fluctuations in HBV DNA levels in the latter patients, there is no absolute cutoff level that is reliable for differentiating patients in the inactive carrier state from those with HBeAg-negative chronic hepatitis B.(86)
Diagnostic algorithms:
Tests for HBV markers are useful in confirming the diagnosis of HBV infection and in the selection and monitoring of patients for antiviral therapy.
Acute hepatitis: The diagnosis of acute hepatitis B is based upon the detection of hepatitis B surface antigen (HBsAg) and IgM hepatitis B core antibody (anti-HBc) (figure 4). During the initial phase of infection, markers of HBV replication, hepatitis B e antigen (HBeAg) and HBV DNA, are also present. Recovery is accompanied by the disappearance of HBV DNA, HBeAg to hepatitis B e antibody (anti-HBe) seroconversion, and subsequently HBsAg to hepatitis B surface antibody (anti-HBs) seroconversion.
Rarely, patients present during the window period when HBsAg has become negative but anti-HBs is not yet positive. In this setting, which is more common in patients with fulminant hepatitis B in whom virus clearance tends to be more rapid, IgM anti-HBc is the sole marker of acute HBV infection (figure 3).
Past HBV infection: Previous HBV infection is characterized by the presence of anti-HBs and IgG anti-HBc (figure 4). Immunity to HBV infection after vaccination is indicated by the presence of anti-HBs only.
Chronic HBV infection: The diagnosis of chronic HBV infection is based upon the persistence of HBsAg for more than six months (figure 4). Additional tests for HBV replication – HBeAg and serum HBV DNA – should be performed to determine if the patient should be considered for antiviral therapy.
All patients with chronic HBV infection should be regularly monitored because HBV DNA and alanine transaminase (ALT) levels vary during the course of infection to monitor for progression of liver disease. In addition, patients who are not candidates for treatment at the time of presentation may become candidates for treatment during follow-up.
HBeAg-negative patients who have normal serum ALT and low (<2000 international units/mL) or undetectable HBV DNA are considered to be in an inactive carrier state. These patients generally have a good prognosis and antiviral treatment is not indicated. However, serial tests are necessary to accurately differentiate them from patients with HBeAg-negative chronic hepatitis who have fluctuating ALT and/or HBV DNA levels. Thus, it is recommended that these patients have repeat ALT +/- HBV DNA tests at three-month intervals during the first year.(87) Patients who are truly inactive carriers should continue to be monitored, but at less frequent intervals. HBeAg-negative patients with elevated serum ALT concentrations should be tested for serum HBV DNA to determine if the liver disease is related to persistent HBV (wild-type or HBeAg-negative variants with mutations in the precore or basal core promoter region that abolish or decrease HBeAg production) replication. Quantification of HBsAg levels can help to differentiate inactive carriers from patients with HBeAg-negative chronic hepatitis.(88) Additional tests for hepatitis C (HCV) and hepatitis D (HDV) should also be performed to rule out superinfection with other hepatitis virus(es).
Occult HBV infection: There exists a subset of patients with occult HBV infection defined as the presence of detectable HBV DNA by polymerase chain reaction (PCR) in patients who are negative for HBsAg. Such patients have been further subclassified as having “seropositive” or “seronegative” HBV depending upon whether they are positive or negative for other HBV markers, most commonly anti-HBc.(89-91) Most of these patients have very low or undetectable serum HBV DNA levels accounting for the failure to detect HBsAg.
PATIENT SELECTION FOR TREATMENT
The decision to initiate treatment is primarily based upon the presence or absence of cirrhosis, the ALT level, and the HBV DNA level. However, there are additional indications for patients with certain concurrent conditions, such as malignancy and pregnancy.
Patients, who are not deemed to be treatment candidates at presentation, and those who decide to defer treatment, should undergo monitoring of liver biochemical tests, HBV DNA, and HBeAg status since liver disease and/or HBV replication may become active later.
THERAPY CONSIDERATION
Patients with hepatitis B disease and fulminant hepatic failure should be hospitalized in the intensive care unit (ICU) and be considered as liver transplant candidates in the event that they do not recover. Any patient with acute HBV disease needs to be treated with first-line oral therapy, such as tenofovir disoproxil fumarate (TDF) or entecavir (ETV). Patients with acute hepatitis should be monitored with blood tests in order to document biochemical improvement.
Patients with acute or chronic hepatitis without cirrhosis have no dietary restrictions. For individuals with decompensated cirrhosis (prominent signs of portal hypertension or encephalopathy), the following dietary limitations are indicated:
- A low-sodium diet (1.5 g/day)
- High-protein diet (ie, white-meat protein, such as chicken, turkey, or fish)
- Fluid restriction (1.5 L/day) in cases of hyponatremia
TREATMENT OPTIONS
Pharmacologic Management
Currently, pegylated interferon alfa (PEG-IFN-a), entecavir (ETV), tenofovir disoproxil fumarate (TDF) and tenofovir Alafenamide (TAF) are the first-line agents in the treatment of hepatitis B disease. These are the main treatment drugs approved globally for this disease, although ongoing trials are investigating new types of medications, such as tenofovir disoproxil in combination with emtricitabine (FTC).
Lamivudine (3TC), telbivudine, and adefovir are of historical interest. These agents are currently considered second- or third-line therapy, or “nonpreferred” treatment.(92)
Acute Hepatitis B:
Treatment of acute HBV depends upon the clinical setting. However, appropriate measures should be taken to prevent infection in all exposed contacts, and hepatitis B immune globulin and hepatitis B vaccine should be administered to all household and sexual contacts who are not known to be immune.
For most patients, treatment is mainly supportive. As a general rule, patients with a severe or a protracted course (e.g. those who develop a coagulopathy [international normalized ratio (INR) >1.5], those with persistent symptoms or marked jaundice [bilirubin >10 mg/dL] for more than four weeks after presentation) should be treated. Patients with acute liver failure due to HBV to reduce the likelihood of reinfection post-liver transplant should also be treated.
For those who require treatment, tenofovir or entecavir are acceptable options given as monotherapy. Treatment can be stopped after confirmation that the patient has cleared HBsAg (two consecutive tests four weeks apart). Lamivudine or telbivudine can also be used, as the duration of treatment is generally short. However, since severe exacerbations of chronic HBV in previously undiagnosed patients can be difficult to differentiate from acute HBV, tenofovir or entecavir are preferred. Adefovir is not typically used because of its weak antiviral activity, and interferon should be avoided because of the risk of bacterial infections and a further increase in hepatic necroinflammation in patients with severe hepatitis or acute liver failure.
Chronic Hepatitis B:
Acute liver failure or decompensated cirrhosis: Patients with life-threatening liver disease secondary to HBV should initiate antiviral therapy. Antiviral treatment also reduces the risk of recurrent HBV should these patients require liver transplantation.
Compensated cirrhosis: Patients with compensated cirrhosis and an HBV DNA >2000 international units/mL (>104 copies/mL) should be treated with antiviral therapy regardless of the HBeAg status or the serum ALT level. Treatment should be considered even if HBV DNA levels are lower than 2000 international units/mL.
Patients without cirrhosis
HBeAg-positive (immune active phase): For HBeAg-positive patients without cirrhosis, treatment should be initiated when the HBV DNA is >20,000 international units/mL (>105 copies/mL) and the ALT is >2 x ULN.(94) Treatment should be delayed for three to six months in newly diagnosed HBeAg-positive patients with compensated liver disease to determine whether spontaneous HBeAg seroconversion will occur.
HBeAg-negative chronic hepatitis: Treatment may be initiated immediately once a diagnosis of HBeAg-negative chronic hepatitis (ALT >2 x ULN and HBV DNA >2000 international units/mL) is established because sustained remission is rare in the absence of treatment. However, delaying treatment for two to three months to allow patients to understand the disease, the need for long-term (and often lifelong) treatment, and the importance of adherence is reasonable in patients with no evidence of cirrhosis.
Patients receiving immunosuppressive therapy: Antiviral therapy should be administered to most patients with chronic HBV prior to initiating immunosuppressive therapy, regardless of the HBV DNA or aminotransferase levels. Such patients are at risk for HBV reactivation if they receive immunosuppressive therapy. The level of risk is influenced by the type of immunosuppressive agent that is used.
Pregnant women: For pregnant women, the indications for antiviral therapy are generally the same as those for patients who are not pregnant. However, women with high viral loads (>2 x 105 international units/mL) should initiate therapy in the third trimester, even if the aminotransferase levels are normal, to prevent transmission to their child.
Patients with hepatocellular carcinoma: All patients with hepatocellular carcinoma (HCC) should be treated with a nucleos(t)ide analogue (e.g. tenofovir or entecavir). Treatment with nucleos(t)ide analogues can reduce the risk of recurrence and improve the prognosis of HBV-related HCC after curative therapy.
Patients with hepatitis C coinfection: Patients who have coinfection with HBV and HCV are at risk for HBV reactivation if they are being treated for HCV with direct-acting antiviral therapy and are not receiving treatment for HBV.(95)
Antiviral therapy:
Treatment strategies for chronic HBV typically include pegylated interferon (PegIFN) or nucleos(t)ide analogs (e.g. entecavir and tenofovir.(96)
Interferon: The main role of interferon is primarily treatment of young patients with well compensated liver disease who do not wish to be on long-term treatment. The advantages of interferon compared to nucleos(t)ide analogues are its finite duration of treatment, the absence of selection of resistant variants, and a more durable response. On the other hand, side effects from interferon are troubling for many patients, and (less commonly) can be severe. Furthermore, interferon should not be used in pregnant women and patients with decompensated disease or compensated cirrhosis and portal hypertension.
Interferon alfa is administered by subcutaneous injection. The preferred formulation is peginterferon alfa-2a, which should be administered as 180 mcg once weekly for 48 weeks for HBeAg-positive or HBeAg-negative chronic HBV.(97) Standard interferon should be used only if PegIFN and nucleos(t)ide analogues are not available.
Nucleos(t)ide analogues: Several nucleos(t)ide analogue agents are available. The available agents include:
Entecavir: The main advantages of entecavir are its potent antiviral activity and low rate of drug resistance in patients who are nucleos(t)ide-naïve (approximately 1 percent with up to five years of treatment). However, entecavir should not be used for patients with lamivudine-resistant HBV, since resistance has been observed in up to 50 percent of lamivudine-refractory patients after five years of treatment. Entecavir is administered orally. For nucleoside-naïve adults and adolescents older than 16, the recommended dose is 0.5 mg once daily. The dose should be increased to 1 mg daily for those with decompensated liver disease. The dose should also be increased to 1 mg daily if it is used for patients who have been treated with lamivudine in the past; however, for such patients, tenofovir is preferred.
Tenofovir: Tenofovir can be used as first-line therapy in treatment-naïve patients and also in those who have had prior exposure, or developed drug resistance, to other nucleos(t)ide analogues (e.g. lamivudine). In clinical trials of patients receiving tenofovir disoproxil fumarate, no signature mutation for tenofovir resistance has been identified, even among those who have been treated for up to eight years. There are two formulations of tenofovir, tenofovir disoproxil fumarate and tenofovir alafenamide. For most patients, tenofovir alafenamide (25 mg daily) is recommended rather than tenofovir disoproxil fumarate (300 mg daily), if available.
Lamivudine: The main advantages of lamivudine are its lower cost compared with the other oral agents and the many years of experience confirming its safety. However, the role of lamivudine in the care of patients with chronic HBV is diminishing given the high rate of drug resistance and the availability of new therapies, such as entecavir and tenofovir, which are associated with lower rates of resistance. The recommended dose of lamivudine for adults with normal renal function without concomitant HIV infection is 100 mg daily. Dose adjustment is required in those with decreased renal function. For patients with HIV, a higher dose (lamivudine 300 mg once daily) is used as part of an HIV antiretroviral regimen.
Adefovir: The most important role of adefovir is in the treatment of patients with lamivudine-resistant HBV, preferably in combination with other agents. However, this role has been replaced by tenofovir, which is more potent and effective when used as monotherapy. If used, adefovir is administered orally, and the dose is 10 mg daily. Patients with impaired renal function should have the dosing interval adjusted.
Telbivudine: Telbivudine is administered orally. The recommended dose is 600 mg once daily. Dose should be adjusted in patients with impaired renal function. Telbivudine appears to have slightly more potent antiviral effects compared with lamivudine and adefovir.
Surgical Intervention
Orthotopic liver transplantation (OLT) is the treatment of choice for patients with fulminant hepatic failure who do not recover and for patients with end-stage liver disease due to hepatitis B disease. The implementation of hepatitis B immunoglobulin (HBIG) during and after the OLT period, and of lamivudine (3TC) or adefovir in the pre- and post-OLT periods, dramatically reduces the recurrence rate of hepatitis B. The current standard is to use HBIG and tenofovir disoproxil fumarate (TDF) or entecavir (ETV). At some centers, HBIG is being stopped at approximately 12 months or sooner; at other centers, HBIG is no longer being used, with the focus on first-line therapy.
Vaccination
Universal hepatitis B vaccination programs are ongoing in endemic areas, with encouraging results. The hepatitis B vaccine consists of recombinant hepatitis B surface antigen (HBsAg) produced in yeast. A series of 3 injections may achieve HBsAg antibody (anti-HBs) levels greater than 10 million IU/mL in approximately 95% of vaccinated individuals. Vaccination with a single dose must be repeated every 5-10 years.
All newborns must be vaccinated against hepatitis B. For infants born to mothers with active hepatitis B, a passive-active approach (hepatitis B immunoglobulin (HBIG) and vaccination) is recommended.
GOALS OF THERAPY
The goals of antiviral therapy are suppression of HBV DNA, loss of HBeAg (in patients who were initially HBeAg-positive), and loss of HBsAg. A sustained viral response, particularly in those who clear both HBeAg and HBsAg, is almost invariably accompanied by normalization of serum ALT, a decrease in necroinflammatory activity, and over time, a decrease in fibrosis as well. Antiviral treatment can also reduce the risk of long-term complications from chronic HBV (e.g. liver failure and hepatocellular carcinoma) as well as the transmission of HBV to others. For some patients, immediate antiviral therapy is indicated, whereas for others, treatment may be deferred with careful monitoring.
GUIDELINES
Various algorithms have been proposed, such as that by the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver Diseases (EASL), the Asian Pacific Association for the Study of the Liver (APASL) and National Institute for Health and Clinical Excellence (NICE).
To review AASLD Guidelines for Treatment of Chronic Hepatitis B, click the below given link:
https://www.aasld.org/publications/practice-guidelines-0
To review EASL Clinical Practice Guidelines: Management of hepatitis B virus infection, click the below given link:
http://www.easl.eu/medias/cpg/management-of-hepatitis-B-virus-infection/English-report.pdf
To review Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update, click the below given link:
http://apasl.info/apasl-hbv-guideline-2016.pdf
To review NICE Guidelines on Hepatitis B (chronic): diagnosis and management, click the below given link:
https://www.nice.org.uk/guidance/cg165
CONSULTATION AND LONG TERM MONITORING
Individuals with inactive chronic infection with the hepatitis B virus (HBV) should have routine blood tests at least annually to check their aminotransferase levels. Patients with chronic active hepatitis should also undergo blood tests (i.e. to evaluate aminotransferase levels, antigen-antibody HBV profile, viral load, and alpha-fetoprotein (AFP) levels), as well as treatment. In rare cases, patients may be considered for liver biopsy.
Current guidelines recommend monitoring of HBV DNA and alanine aminotransferase (ALT) levels at least annually. Patients with cirrhosis must be monitored for hepatocellular carcinoma (HCC) by having their AFP levels checked every 6-12 months and undergoing surveillance with abdominal ultrasonography.(98)
Carriers of HBV should be counseled regarding the risk of transmission to others. Patients should be advised regarding prevention of sexual transmission (i.e. vaccination of spouses and steady sex partners in individuals with monogamous partners, and safe sex practice including use of condoms in subjects with multiple partners), perinatal transmission, and risk of environmental exposure from blood.
PRECAUTIONS
Pre-exposure vaccination: Vaccination against hepatitis B virus (HBV) prior to an exposure is the best way to prevent HBV infection. Universal vaccination of newborns is recommended in most countries. Vaccination should also be provided to individuals who are not immune to HBV and are at high risk of exposure or a poor disease outcome (e.g. health care personnel, injection drug users, household contacts of hepatitis B surface antigen [HBsAg]-positive patients, men who have sex with men, human immunodeficiency virus [HIV]-infected patients, hepatitis C virus [HCV]-infected patients).
Postexposure prophylaxis: Postexposure prophylaxis to prevent HBV infection should be considered for individuals who have had an exposure that could potentially transmit HBV. These include percutaneous (e.g. bite or needlestick) or mucosal exposures to blood or infectious secretions (e.g. semen, body fluids that contain blood) of a patient who is HBsAg positive or whose HBsAg status is unknown.
Management of special populations: Pre-exposure hepatitis B vaccination is the best way to prevent HBV transmission. However, for certain groups of patients, additional strategies are also used. An overview of these strategies are provided below.
Mother-to-child transmission: To reduce mother-to-child transmission, infants born to mothers who are HBsAg positive should receive active and passive immunization (i.e. hepatitis B vaccine and hepatitis B immune globulin [HBIG]) as soon as possible and preferably within 12 hours of birth. In addition, administering antiviral therapy to mothers with high HBV viral loads can further reduce the risk of infection in the newborn.
Sexual exposure: HBsAg-positive patients should use condoms to reduce the risk of sexual transmission of HBV if their partner is not immune or if their partner’s immune status is unknown. In addition, spouses and steady sex partners of a patient with chronic HBV should be screened to see if they have been previously infected. Those who are not immune and are without evidence of chronic HBV should be vaccinated and immunity verified by testing for anti-HBs one to two months after completing the course of vaccination.
Percutaneous: To prevent percutaneous transmission of HBV (via drug use or piercings), patients should be educated about the use of sterile disposable needles and equipment.
Health care providers: Health care providers (HCP) should be immunized against HBV. In addition, HCP should be trained in techniques to minimize the risk of exposure to bloodborne pathogens.
Transplant recipients: To help prevent HBV transmission to solid and hematopoietic transplant recipients, donors are routinely screened for HBsAg. In some countries, donors are also screened for anti-HBc and HBV DNA.
REFERENCES
- Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012; 30:2212.
- Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015; 386:1546.
- Zhang Q, Qi W, Wang X, et al. Epidemiology of Hepatitis B and Hepatitis C Infections and Benefits of Programs for Hepatitis Prevention in Northeastern China: A Cross-Sectional Study. Clin Infect Dis 2016; 62:305.
- Noorali S, Hakim ST, McLean D, Kazmi SU, Bagasra O. Prevalence of Hepatitis B virus genotype D in females in Karachi, Pakistan. J Infect Developing Countries. 2008;2:373–378.
- Hakim ST, Kazmi SU, Bagasra O. Seroprevalence of Hepatitis B and C Genotypes Among Young Apparently Healthy Females of Karachi-Pakistan. Libyan J Med. 2008;3:66–70. doi: 10.4176/071123
- Hepatitis prevention & control program Sindh (chief minister’s initiative) 2009. directorate general health services, Hyderabad, Sindh, Pakistan
- Nikolaos T Pyrsopoulos. Hepatitis B [Internet]. Medscape; 2018 [cited 2018 November 19]. Available from: https://emedicine.medscape.com/article/177632-overview#a3
- Blumberg BS. Australia antigen and the biology of hepatitis B. Science. 1977 Jul 1. 197(4298):17-25.
- Norder H, Courouce AM, Magnius LO. Complete genomes, phylogenetic relatedness, and structural proteins of six strains of the hepatitis B virus, four of which represent two new genotypes. Virology. 1994 Feb. 198(2):489-503.
- Lau JY, Wright TL. Molecular virology and pathogenesis of hepatitis B. Lancet. 1993 Nov 27. 342(8883):1335-40.
- Chisari FV, Ferrari C. Hepatitis B virus immunopathology. Springer Semin Immunopathol. 1995. 17(2-3):261-81.
- Davies SE, Portmann BC, O’Grady JG, et al. Hepatic histological findings after transplantation for chronic hepatitis B virus infection, including a unique pattern of fibrosing cholestatic hepatitis. Hepatology. 1991 Jan. 13(1):150-7.
- Gish RG, Locarnini S. Chronic hepatitis B viral infection. Yamada T, ed. Textbook of Gastroenterology. 5th ed. Oxford, UK: Blackwell Publishing; 2009. 2112-38.
- Jung MC, Diepolder HM, Pape GR. T cell recognition of hepatitis B and C viral antigens. Eur J Clin Invest. 1994 Oct. 24(10):641-50.
- Chisari FV. Cytotoxic T cells and viral hepatitis. J Clin Invest. 1997 Apr 1. 99(7):1472-7.
- Chang MH, Chen CJ, Lai MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997 Jun 26. 336(26):1855-9
- Fattovich G, Giustina G, Schalm SW, et al. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology. 1995 Jan. 21(1):77-82
- Yu MC, Yuan JM, Ross RK, Govindarajan S. Presence of antibodies to the hepatitis B surface antigen is associated with an excess risk for hepatocellular carcinoma among non-Asians in Los Angeles County, California. Hepatology. 1997 Jan. 25(1):226-8
- Kuo A, Gish R. Chronic hepatitis B infection. Clin Liver Dis. 2012 May. 16(2):347-69
- Tong W, He J, Sun L, He S, Qi Q. Hepatitis B virus with a proposed genotype I was found in Sichuan Province, China. J Med Virol. 2012 Jun. 84(6):866-70.
- Sonneveld MJ, Rijckborst V, Zeuzem S, et al. Presence of precore and core promoter mutants limits the probability of response to peginterferon in hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2012 Jul. 56(1):67-75.
- Alter MJ, Hadler SC, Margolis HS, et al. The changing epidemiology of hepatitis B in the United States. Need for alternative vaccination strategies. JAMA 1990; 263:1218.
- Beasley RP, Hwang LY, Lin CC, et al. Incidence of hepatitis B virus infections in preschool children in Taiwan. J Infect Dis 1982; 146:198
- Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med 1975; 292:771
- Lin CL, Kao JH, Chen BF, et al. Application of hepatitis B virus genotyping and phylogenetic analysis in intrafamilial transmission of hepatitis B virus. Clin Infect Dis 2005; 41:1576
- Ahmed MM, Huang TH, Xie QD. A sensitive and rapid assay for investigating vertical transmission of hepatitis B virus via male germ line using EGFP Vector as reporter. J Biomed Biotechnol 2008; 2008:495436.
- Ahmed MM, Huang TH, Xie QD. An improved experimental model for studying vertical transmission of hepatitis B virus via human spermatozoa. J Virol Methods 2008; 151:116.
- Huang JM, Huang TH, Qiu HY, et al. Effects of hepatitis B virus infection on human sperm chromosomes. World J Gastroenterol 2003; 9:736.
- Hadchouel M, Scotto J, Huret JL, et al. Presence of HBV DNA in spermatozoa: a possible vertical transmission of HBV via the germ line. J Med Virol 1985; 16:61
- World Health Organization. Guidelines on assessing donor suitability for blood donation. http://apps.who.int/iris/bitstream/handle/10665/76724/9789241548519_eng.pdf?sequence=1&isAllowed=y (Accessed on November 19, 2018).
- Goh KT. Prevention and control of hepatitis B virus infection in Singapore. Ann Acad Med Singapore 1997; 26:671.
- Centers for Disease Control. Surveillance for viral hepatitis – United States, 2013 http://www.cdc.gov/hepatitis/statistics/2013surveillance/commentary.htm#hepatitisB (Accessed on November 19, 2018).
- Iqbal K, Klevens RM, Kainer MA, et al. Epidemiology of Acute Hepatitis B in the United States From Population-Based Surveillance, 2006-2011. Clin Infect Dis 2015; 61:584
- World Health Organization. Hepatitis B fact sheet. http://www.who.int/mediacentre/factsheets/fs204/en/ (Accessed on November 19, 2018).
- Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011; 378:571.
- Hagan H, McGough JP, Thiede H, et al. Syringe exchange and risk of infection with hepatitis B and C viruses. Am J Epidemiol 1999; 149:203.
- Des Jarlais DC, Diaz T, Perlis T, et al. Variability in the incidence of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus infection among young injecting drug users in New York City. Am J Epidemiol 2003; 157:467.
- Bialek SR, Bower WA, Mottram K, et al. Risk factors for hepatitis B in an outbreak of hepatitis B and D among injection drug users. J Urban Health 2005; 82:468.
- Thompson ND, Perz JF, Moorman AC, Holmberg SD. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998-2008. Ann Intern Med 2009; 150:33.
- Gerberding JL. The infected health care provider. N Engl J Med 1996; 334:594.
- Williams IT, Perz JF, Bell BP. Viral hepatitis transmission in ambulatory health care settings. Clin Infect Dis 2004; 38:1592.
- Goh KT, Ding JL, Monteiro EH, Oon CJ. Hepatitis B infection in households of acute cases. J Epidemiol Community Health 1985; 39:123.
- Anna SF Lok. Hepatitis B virus: Clinical manifestations and natural history [Internet]. Uptodate; 2018 [cited 2018 November 19]. Available from: https://www.uptodate.com/contents/hepatitis-b-virus-clinical-manifestations-and-natural-history?search=hepatitis%20B%20natural%20history&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Hoofnagle JH, Dusheiko GM, Seeff LB, et al. Seroconversion from hepatitis B e antigen to antibody in chronic type B hepatitis. Ann Intern Med 1981; 94:744.
- Realdi G, Alberti A, Rugge M, et al. Seroconversion from hepatitis B e antigen to anti-HBe in chronic hepatitis B virus infection. Gastroenterology 1980; 79:195.
- Lok AS. Natural history and control of perinatally acquired hepatitis B virus infection. Dig Dis 1992; 10:46
- Chang MH, Hwang LY, Hsu HC, et al. Prospective study of asymptomatic HBsAg carrier children infected in the perinatal period: clinical and liver histologic studies. Hepatology 1988; 8:374.
- Lok AS, Lai CL. A longitudinal follow-up of asymptomatic hepatitis B surface antigen-positive Chinese children. Hepatology 1988; 8:1130
- Hsu HY, Chang MH, Hsieh KH, et al. Cellular immune response to HBcAg in mother-to-infant transmission of hepatitis B virus. Hepatology 1992; 15:770
- Liaw YF, Chu CM, Lin DY, et al. Age-specific prevalence and significance of hepatitis B e antigen and antibody in chronic hepatitis B virus infection in Taiwan: a comparison among asymptomatic carriers, chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. J Med Virol 1984; 13:385.
- Lok AS, Lai CL, Wu PC, et al. Spontaneous hepatitis B e antigen to antibody seroconversion and reversion in Chinese patients with chronic hepatitis B virus infection. Gastroenterology 1987; 92:1839.
- Maruyama T, Iino S, Koike K, et al. Serology of acute exacerbation in chronic hepatitis B virus infection. Gastroenterology 1993; 105:1141
- Hsu HC, Su IJ, Lai MY, et al. Biologic and prognostic significance of hepatocyte hepatitis B core antigen expressions in the natural course of chronic hepatitis B virus infection. J Hepatol 1987; 5:45
- Lai M, Hyatt BJ, Nasser I, et al. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol 2007; 47:760.
- Kumar M, Sarin SK, Hissar S, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology 2008; 134:1376.
- Papatheodoridis GV, Manolakopoulos S, Liaw YF, Lok A. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: a systematic review. J Hepatol 2012; 57:196.
- Bonino F, Rosina F, Rizzetto M, et al. Chronic hepatitis in HBsAg carriers with serum HBV-DNA and anti-HBe. Gastroenterology 1986; 90:1268.
- Lok AS, Hadziyannis SJ, Weller IV, et al. Contribution of low level HBV replication to continuing inflammatory activity in patients with anti-HBe positive chronic hepatitis B virus infection. Gut 1984; 25:1283.
- Carman WF, Jacyna MR, Hadziyannis S, et al. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet 1989; 2:588.
- Lok AS, Akarca U, Greene S. Mutations in the pre-core region of hepatitis B virus serve to enhance the stability of the secondary structure of the pre-genome encapsidation signal. Proc Natl Acad Sci U S A 1994; 91:4077.
- Okamoto H, Tsuda F, Akahane Y, et al. Hepatitis B virus with mutations in the core promoter for an e antigen-negative phenotype in carriers with antibody to e antigen. J Virol 1994; 68:8102.
- Brunetto MR, Giarin MM, Oliveri F, et al. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc Natl Acad Sci U S A 1991; 88:4186.
- Chung HT, Lai CL, Lok AS. Pathogenic role of hepatitis B virus in hepatitis B surface antigen-negative decompensated cirrhosis. Hepatology 1995; 22:25.
- Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology 2002; 123:1084.
- Yuen MF, Wong DK, Sablon E, et al. HBsAg seroclearance in chronic hepatitis B in the Chinese: virological, histological, and clinical aspects. Hepatology 2004; 39:1694.
- Huo TI, Wu JC, Lee PC, et al. Sero-clearance of hepatitis B surface antigen in chronic carriers does not necessarily imply a good prognosis. Hepatology 1998; 28:231.
- Yuen MF, Wong DK, Fung J, et al. HBsAg Seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology 2008; 135:1192.
- Kim GA, Lee HC, Kim MJ, et al. Incidence of hepatocellular carcinoma after HBsAg seroclearance in chronic hepatitis B patients: a need for surveillance. J Hepatol 2015; 62:1092.
- Liaw YF, Tsai SL, Sheen IS, et al. Clinical and virological course of chronic hepatitis B virus infection with hepatitis C and D virus markers. Am J Gastroenterol 1998; 93:354
- Lok AS, Lai CL. alpha-Fetoprotein monitoring in Chinese patients with chronic hepatitis B virus infection: role in the early detection of hepatocellular carcinoma. Hepatology 1989; 9:110
- Anna SF Lok. Hepatitis B virus: Screening and diagnosis [Internet]. Uptodate; 2018 [cited 2018 November 19]. Available from: https://www.uptodate.com/contents/hepatitis-b-virus-screening-and-diagnosis?search=hepatitis%20b%20diagnosis&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018; 67:1560
- Maruyama T, Schödel F, Iino S, et al. Distinguishing between acute and symptomatic chronic hepatitis B virus infection. Gastroenterology 1994; 106:1006
- Chu CM, Liaw YF, Pao CC, Huang MJ. The etiology of acute hepatitis superimposed upon previously unrecognized asymptomatic HBsAg carriers. Hepatology 1989; 9:452
- Tassopoulos NC, Papaevangelou GJ, Sjogren MH, et al. Natural history of acute hepatitis B surface antigen-positive hepatitis in Greek adults. Gastroenterology 1987; 92:1844
- Hendrickson B, Kamili S, Timmons T, et al. Notes from the Field: False-Negative Hepatitis B Surface Antigen Test Results in a Hemodialysis Patient – Nebraska, 2017. MMWR Morb Mortal Wkly Rep 2018; 67:311
- Servant-Delmas A, Mercier-Darty M, Ly TD, et al. Variable capacity of 13 hepatitis B virus surface antigen assays for the detection of HBsAg mutants in blood samples. J Clin Virol 2012; 53:338
- Okada K, Kamiyama I, Inomata M, et al. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med 1976; 294:746.
- Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol 1977; 105:94.
- Hwang LY, Roggendorf M, Beasley RP, Deinhardt F. Perinatal transmission of hepatitis B virus: role of maternal HBeAg and anti-HBc IgM. J Med Virol 1985; 15:265
- Alter HJ, Seeff LB, Kaplan PM, et al. Type B hepatitis: the infectivity of blood positive for e antigen and DNA polymerase after accidental needlestick exposure. N Engl J Med 1976; 295:909
- Michalak TI, Pasquinelli C, Guilhot S, Chisari FV. Hepatitis B virus persistence after recovery from acute viral hepatitis. J Clin Invest 1994; 93:230
- Lok AS, Chung HT, Liu VW, Ma OC. Long-term follow-up of chronic hepatitis B patients treated with interferon alfa. Gastroenterology 1993; 105:1833
- Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000–summary of a workshop. Gastroenterology 2001; 120:1828
- Martinot-Peignoux M, Boyer N, Colombat M, et al. Serum hepatitis B virus DNA levels and liver histology in inactive HBsAg carriers. J Hepatol 2002; 36:543
- Chu CJ, Hussain M, Lok AS. Quantitative serum HBV DNA levels during different stages of chronic hepatitis B infection. Hepatology 2002; 36:1408
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507
- Chan HL, Thompson A, Martinot-Peignoux M, et al. Hepatitis B surface antigen quantification: why and how to use it in 2011 – a core group report. J Hepatol 2011; 55:1121
- Conjeevaram HS, Lok AS. Occult hepatitis B virus infection: a hidden menace? Hepatology 2001; 34:204.
- Bréchot C, Thiers V, Kremsdorf D, et al. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology 2001; 34:194.
- Minuk GY, Sun DF, Uhanova J, et al. Occult hepatitis B virus infection in a North American community-based population. J Hepatol 2005; 42:480.
- Mutimer D, Naoumov N, Honkoop P, et al. Combination alpha-interferon and lamivudine therapy for alpha-interferon-resistant chronic hepatitis B infection: results of a pilot study. J Hepatol. 1998 Jun. 28(6):923-9
- Caputo R, Gelmetti C, Ermacora E, Gianni E, Silvestri A. Gianotti-Crosti syndrome: a retrospective analysis of 308 cases. J Am Acad Dermatol. 1992 Feb. 26(2 Pt 1):207-10.
- Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016; 63:261
- FDA Drug Safety Communication: FDA warns about the risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C. http://www.fda.gov/Drugs/DrugSafety/ucm522932.htm (Accessed on November 23, 2016).
- Lok AS, McMahon BJ, Brown RS Jr, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology 2016; 63:284
- Liaw YF, Leung N, Guan R, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int 2005; 25:472
El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how?. Therap Adv Gastroenterol. 2011 Jan. 4(1):5-10.
