EPIDEMIOLOGY
Hepatitis A virus (HAV) has a worldwide distribution, particularly in resource-poor regions.(1-3) Globally, an estimated 1.4 million cases occur each year.(4)
Between 1995 and 2006, the reported hepatitis A incidence declined by 90% to the lowest rate ever recorded, 1.2 cases per 100,000 population.(5) The greatest reductions were seen in children and in those regions where routine vaccination of children is observed.
The highest seropositivity (i.e. the highest prevalence of antibody to HAV) is observed in adults in urban Africa, Asia, and South America, where evidence of past infection is nearly universal.(6-10)
PATHOPHYSIOLOGY
HAV is a single-stranded, positive-sense, linear RNA enterovirus of the Picornaviridae family. In humans, viral replication depends on hepatocyte uptake and synthesis, and assembly occurs exclusively in liver cells.
Various genotypes of HAV exist; however, there appears to be only 1 serotype. Virion proteins 1 and 3 are the primary sites of antibody recognition and subsequent neutralization. No antibody cross-reactivity has been identified with other viruses causing acute hepatitis. Evidence in recent years appears to show exosomes playing a dual role in transmission of HAV and HCV, allowing these viruses to evade antibody-mediated immune responses but, paradoxically, also be detected by plasmacytoid dendritic cells (pDCs) leading to innate immune activation and type I interferon production.(11)
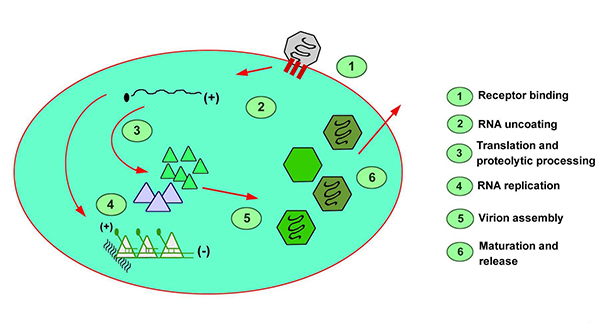
Hepatocyte uptake involves a receptor, identified by Kaplan et. al., on the plasma membrane of the cell, and viral replication is believed to occur exclusively in hepatocytes.(12) The demonstration of HAV in saliva has raised questions about this exclusivity. After entry into the cell, viral RNA is uncoated, and host ribosomes bind to form polysomes. Viral proteins are synthesized, and the viral genome is copied by a viral RNA polymerase. Assembled virus particles are shed into the biliary tree and excreted in the feces.
Hepatitis A (picture courtesy Medscape)
Minimal cellular morphologic changes result from hepatocyte infection. The development of an immunologic response to infection is accompanied by a predominantly portal and periportal lymphocytic infiltrate and a varying degree of necrosis. The hepatocyte injury is secondary to the host’s immunologic response. This hypothesis is supported by the lack of cytotoxic activity in tissue culture and correlations between immunologic response and manifestations of hepatocyte injury.
The incubation period usually lasts 2-6 weeks, and the time to onset of symptoms may be dose related. The presence of disease manifestations and the severity of symptoms after HAV infection directly correlate with patient age. In developing nations, the age of acquisition is before age 2 years. In Western societies, acquisition is most frequent in persons aged 5-17 years. Within this age range, the illness is more often mild or subclinical; however, severe disease, including fulminant hepatic failure, does occur.
TRANSMISSION
Hepatitis A virus is usually transmitted via the fecal-oral route, either via person-to-person contact or consumption of contaminated food or water
Person-to-person contact
✔ Transmission within households
✔ Sexual transmission
✔ Residential institution transmission
✔ Daycare center transmission
✔ Transmission among military personnel
Contact with contaminated food or water
✔ Consumption of raw or undercooked shellfish, vegetables, or other foods
✔ Consumption of foods contaminated by infected food handlers
- Blood transfusion
- Illicit drug use
NATURAL HISTORY(13)
Along with outlining the presenting complaint and its severity and sequelae, the history should also initiate a search for the source of exposure (e.g. overseas travel, lack of immunization, intravenous (IV) drug use) and attempt to exclude other possible causes of acute hepatitis (e.g. accidental acetaminophen overdose). The incubation period is 2-6 weeks (mean, 4 week). Shorter incubation periods may result from higher total dose of the viral inoculum.
Prodrome
In the prodrome, patients may have mild flulike symptoms of anorexia, nausea and vomiting, fatigue, malaise, low-grade fever (usually < 39.5°C), myalgia, and mild headache. Smokers often lose their taste for tobacco, like persons presenting with appendicitis.
Icteric phase
In the icteric phase, dark urine appears first (bilirubinuria). Pale stool soon follows, although this is not universal. Jaundice occurs in most (70-85%) adults with acute HAV infection; it is less likely in children and is uncommon in infants. The degree of icterus also increases with age. Abdominal pain occurs in approximately 40% of patients. Itching (pruritus), although less common than jaundice, is generally accompanied by jaundice.
Arthralgias and skin rash, although also associated with acute HAV infection, are less frequent than the above symptoms. Rash more often occurs on the lower limbs and may have a vasculitic appearance.
Relapsing hepatitis A
Relapsing hepatitis A is an uncommon sequela of acute infection, is more common in elderly persons, and is characterized by a protracted course of symptoms of the disease and a relapse of symptoms and signs following apparent resolution.
The physical examination focuses on detecting features that support a diagnosis of acute hepatitis and should include an assessment of features of chronic liver disease and, similarly, assessment of any evidence of decompensation.
Hepatomegaly is common. Jaundice or scleral icterus may occur. Patients may have a fever with temperatures of up to 40°C.
SIGN AND SYMPTOMS(17)
Typical manifestations
Acute HAV infection in adults is usually a self-limited illness; fulminant hepatic failure occurs in less than 1 percent of cases. The incubation period of hepatitis A infection averages 28 days (range 15 to 50 days).(14)
More than 70 percent of adults with HAV have symptomatic illness, which begins with abrupt onset of nausea, vomiting, anorexia, fever, malaise, and abdominal pain (see below figure).(15) Within a few days to a week, dark urine (bilirubinuria) appears; pale stools (lacking bilirubin pigment) may also be observed. These are followed by jaundice and pruritus (40 to 70 percent of cases). The early signs and symptoms usually diminish when jaundice appears, and jaundice typically peaks within two weeks.
Timeline for hepatitis A manifestations(16,17)
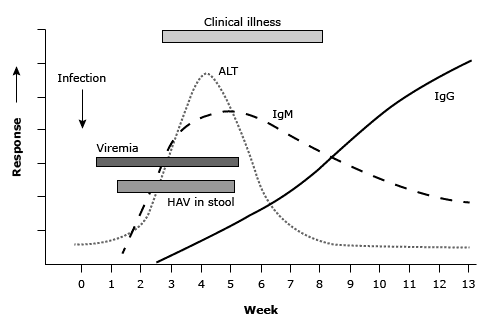
ALT: alanine transaminase; HAV: hepatitis A virus; Ig: immunoglobulin.
Physical findings include fever, jaundice, scleral icterus, hepatomegaly (80 percent of cases), and abdominal pain.(18,19) Less common findings include splenomegaly and extrahepatic manifestations such as skin rash and arthralgias.
Laboratory abnormalities include elevations of serum aminotransferases (often >1000 international units/dL), serum bilirubin (typically ≤10 mg/dL), and alkaline phosphatase (up to 400 U/L) [40]. The serum aminotransferase elevations precede the bilirubin elevation. Serum alanine aminotransferase is commonly higher than the serum aspartate aminotransferase. Serum aminotransferases peak approximately one month after exposure to the virus and then decline by approximately 75 percent per week.(20) The serum bilirubin concentration usually declines within two weeks of peak levels.(18) Other laboratory abnormalities include elevations of acute-phase reactants and inflammatory markers.
Infected individuals are contagious during the incubation period and remain so for about a week after jaundice appears.(21) HAV replicates in the liver and is shed in the stool in high concentrations from two to three weeks before to one week after onset of clinical illness (see figure Timeline for hepatitis A manifestations).(22)
Full clinical and biochemical recovery is observed within two to three months in 85 percent of patients, and complete recovery is observed by six months in nearly all patients.(20) HAV infection does not become chronic, and individuals cannot become reinfected after recovering from infection.
Fulminant hepatic failure refers to the development of severe acute liver injury with encephalopathy and impaired synthetic function (international normalized ratio ≥1.5). It occurs most commonly in individuals >50 years of age and individuals with other liver diseases such as hepatitis B or C.(23)
Extrahepatic manifestations
Extrahepatic manifestations occur most commonly in patients who have protracted illness such as relapsing or cholestatic hepatitis.(24,25)
The most common extrahepatic manifestations include evanescent rash and arthralgias (occurring in 10 to 15 percent of patients). Other conditions related to immune complex disease and vasculitis also occur, including:(24-29)
- Leukocytoclastic vasculitis (most often apparent on the legs and buttocks; biopsy demonstrates anti-HAV immunoglobulin (Ig)M and complement in the blood vessel walls)
- Arthritis
- Glomerulonephritis
- Cryoglobulinemia
- Optic neuritis
- Transverse myelitis
- Toxic epidermal necrolysis
- Myocarditis
- Thrombocytopenia
- Aplastic anemia
- Red cell aplasia
Complications
Complications of acute hepatitis A infection include cholestatic hepatitis, relapsing hepatitis, and autoimmune hepatitis.(26)
Cholestatic hepatitis: Prolonged cholestasis is characterized by a protracted period of jaundice (lasting >3 months); it occurs among fewer than 5 percent of patients with acute hepatitis A infection.(30,31)
The course of cholestatic hepatitis is usually characterized by marked jaundice, pruritus, fever, weight loss, diarrhea, and malaise.(19,26,30,32) Laboratory findings include markedly elevated serum bilirubin (often >10 mg/dL) and alkaline phosphatase, modest elevation of serum aminotransferases, and elevated serum cholesterol. Peak bilirubin levels may be reached in the eighth week or later.
In general, cholestatic hepatitis resolves spontaneously with no sequelae; recognition is important to avoid unnecessary testing. Ultrasonography is appropriate to exclude biliary obstruction; cholangiography or liver biopsy are usually not necessary.(30)
Relapsing hepatitis: Up to 10 percent of patients experience a relapse of symptoms during the six months after acute illness.(22,26,33-37) The duration of clinical relapse is generally less than three weeks, although biochemical relapse may last as long as 12 months.(37) The cause of relapsing hepatitis is unknown, and no predisposing factors for relapse have been identified.(33)
The clinical course usually consists of apparent clinical recovery after acute infection with near normalization of the serum aminotransferases, followed by biochemical (and, in some cases, clinical) relapse; clinical manifestations of relapse are often milder than the initial episode.(33) Serum aminotransferases may exceed 1000 international units/dL, and serum anti-HAV IgM antibodies typically persist throughout the course of the disease.(33,38) HAV can be recovered from stool during relapse episodes, so such patients should be considered infectious.(37)
In general, patients with relapsing hepatitis have complete recovery; recognition is important to avoid unnecessary testing. Ultrasonography is appropriate to exclude biliary obstruction in patients with significant jaundice; cholangiography or liver biopsy are usually not necessary.
Autoimmune hepatitis: Rarely, HAV infection may serve as a trigger for development of autoimmune hepatitis in susceptible individuals.(39,40) Autoimmune hepatitis is a chronic hepatitis characterized by hyperglobulinemia, the presence of circulating autoantibodies (such as antinuclear, antismooth muscle, and/or antiactin antibodies), and inflammatory changes on liver histology.
DIAGNOSTIC TESTS
The diagnosis of acute HAV infection should be suspected in patients with abrupt onset of prodromal symptoms (nausea, anorexia, fever, malaise, or abdominal pain) and jaundice or elevated serum aminotransferase levels, particularly in the setting of known risk factors for hepatitis A transmission.(41)
Nucleic acid testing (NAT) – is the gold standard for diagnosis of viremic stages of hepatitis infection.(42)
Liver biopsy- has a minimal role in acute HAV infection. It may play a part in chronic relapsing HAV infection or in situations where the diagnosis is uncertain. Other investigations (e.g. serum acetaminophen) may be necessary, depending on findings from the history and clinical examination. Molecular diagnostic techniques performed on blood and feces for HAV RNA are purely research tools at present.
Complete blood count- Mild lymphocytosis is not uncommon. Pure red cell aplasia and pancytopenia may rarely accompany infection. Indices of low-grade hemolysis are not uncommon.
Prothrombin time- The prothrombin time (PT) usually remains within or near the reference range. Significant rises should raise concern and support closer monitoring. In the presence of encephalopathy, an elevated PT has ominous implications (e.g. fulminant hepatic failure (FHF)).
Liver enzymes – Rises in the levels of ALT and aspartate aminotransferase (AST) are sensitive for hepatitis A. Levels may exceed 10,000 mIU/mL, with ALT levels generally greater than AST levels. levels usually return to reference ranges over 5-20 weeks. Rises in alkaline phosphatase accompany the acute disease and may progress during the cholestatic phase of the illness following the rises in transaminase levels.
Hepatic synthetic function- Bilirubin level rises soon after the onset of bilirubinuria and follows rises in ALT and AST levels. Levels may be impressively high and can remain elevated for several months; persistence beyond 3 months indicates cholestatic HAV infection.
Older individuals have higher bilirubin levels. Both direct and indirect fractions increase because of hemolysis, which often occurs in acute HAV infection.
Modest falls in serum albumin level may accompany the illness.
Serologic Tests
Anti-hepatitis A virus immunoglobulin M – The diagnosis of acute HAV infection is based on serologic testing for immunoglobulin M (IgM) antibody to HAV. Test results for anti-HAV IgM are positive at the time of onset of symptoms and usually accompany the first rise in the alanine aminotransferase (ALT) level.
This test is sensitive and specific, and the results remain positive for 3-6 months after the primary infection and for as long as 12 months in 25% of patients. In patients with relapsing hepatitis, IgM persists for the duration of this pattern of disease. False-positive results are uncommon and should be considered in the event that anti-HAV IgM persists.
Anti-hepatitis A virus immunoglobulin G – Anti-HAV immunoglobulin G (IgG) appears soon after IgM and generally persists for many years. The presence of anti-HAV IgG in the absence of IgM indicates past infection or vaccination rather than acute infection. IgG provides protective immunity.
THERAPY CONSIDERATION
Patients at risk of developing acute hepatitis A virus (HAV) infection should undergo immunization for the virus. In addition, immunization of those at greater risk for morbidity from acute HAV infection is important.
Treatment generally involves supportive care, with specific complications treated as appropriate. Liver transplantation, in selected cases, is an option if the patient has fulminant hepatic failure (FHF).
TREATMENT OPTIONS
HAV infection is usually self-limited, and treatment consists of supportive care.
Immunization
Vaccination is highly effective at preventing HAV disease. The efficacy of the hepatitis A vaccine ranges from 80% to 100% after 1-2 doses compared to placebo.
Immunization is indicated for individuals traveling to areas of high endemicity who have less than 2 weeks before departure. Both the vaccination and intramuscular (IM) immunoglobulin should be administered to provide long-term immunity, particularly in persons who intend to travel to these areas repeatedly.
People with chronic liver disease of any cause should consider hepatitis A vaccination. Response rates in patients with advanced liver disease and in those on immunosuppressive therapies are likely to be lower. The potentially disastrous outcome of acute HAV infection in this group cannot be overemphasized.
Hepatitis A vaccine is used for active immunization against disease caused by HAV.
Hepatitis A vaccine, inactivated, and hepatitis B vaccine – This combined hepatitis A–hepatitis B vaccine is used for active immunization of persons older than 18 years against disease caused by HAV and infection by all known subtypes of hepatitis B virus (HBV).
Hepatitis A vaccine, inactivated- Hepatitis A vaccine may be administered with immunoglobulin injections without affecting efficacy. Hepatitis A vaccine may be administered with immunoglobulin injections without affecting efficacy.
Immune globulin IM
Immune globulin IM neutralizes circulating myelin antibodies through anti-idiotypic antibodies; down-regulates proinflammatory cytokines, including interferon-gamma; blocks Fc receptors on macrophages; suppresses inducer T and B cells and augments suppressor T cells; blocks the complement cascade; promotes remyelination; and may increase cerebrospinal fluid immunoglobulin G (10%). It is effective when administered within 14 days of exposure.
If the patient is likely to be returning to areas of high endemicity, concurrent vaccination is recommended. For situations in which exposure is likely to occur before vaccination would be effective, both agents may be administered without reducing the efficacy of the HAV vaccine.
Supportive Care
For acute cases of HAV infection, therapy is generally supportive, with no specific treatment of acute uncomplicated illness. Locating the primary source and preventing further outbreaks are paramount. Initial therapy often consists of bed rest. The patient should probably not work during the acute phase of the illness.
Nausea and vomiting are treated with antiemetics. Dehydration may be managed with hospital admission and intravenous (IV) fluids. In most instances, hospitalization is unnecessary. The majority of children have minimal symptoms; adults are more likely to require more intensive care, including hospitalization.
The advent of new antiviral agents, such as direct-acting antivirals (DAAs) and host-targeting agents (HTAs), has expanded the potential therapeutic options available against HAV.(43)
Liver Transplantation:
Patients with fulminant hepatic failure (FHF) are considered for liver transplantation. Recurrent disease after liver transplantation has not been reported. Patient selection for liver transplantation may be difficult, in that 60% of patients recover from FHF without needing the transplant (much as with acetaminophen toxicity), and predicting who needs this life-saving procedure is difficult.
Late referral has ominous implications, with the accompanying comorbidities (e.g. renal failure, coagulopathy, cerebral edema) and waiting times contributing to poor outcomes.
Liver transplantation for chronic relapsing HAV infection has occurred in the context of decompensation with good results; however, there is a report of clinical recurrence after liver transplantation.
Postexposure Prophylaxis
Passive immunization with Immune Globulin Infusion reduces infection when administered within 14 days of exposure (i.e. postexposure prophylaxis). Recommendations for providing postexposure prophylaxis are developed on the basis of risk. Postexposure prophylaxis is recommended for non-immunized close contacts of those recently diagnosed with acute HAV infection.
Medications
The goals of pharmacotherapy are to reduce morbidity and to prevent complications. Agents used include analgesics, antiemetics, vaccines, and immunoglobulins.
Analgesic agents (Acetaminophen): Pain control is essential to quality patient care. Acetaminophen is useful for pain and/or fever. Acetaminophen reduces fever by acting directly on hypothalamic heat-regulating centers, thereby increasing dissipation of body heat via vasodilation and sweating. It relieves mild to moderate pain. Acetaminophen may be safely used to treat some of the symptoms associated with hepatitis A virus (HAV) infection; the dosage should be no higher than 4 g/day.
Antiemetics (Metoclopramide): Metoclopramide is a dopamine antagonist that stimulates acetylcholine release in the myenteric plexus. It acts centrally on chemoreceptor triggers in the floor of the fourth ventricle, and this action provides important antiemetic activity.
GOALS OF THERAPY
The goals of pharmacotherapy are to provide symptomatic relief and to reduce morbidity and prevent complications.
GUIDELINES
The World Health Organization (WHO) has issued a guideline for treatment of HAV and its control and prevention. To review, click on the link below.
http://www.who.int/news-room/fact-sheets/detail/hepatitis-a
To review American Family Physician guidelines on Hepatitis A, please click on below link:
https://www.aafp.org/afp/2012/1201/p1027.html
To review Centers for Disease Control and prevention (CDC) guidelines on Hepatitis A, please click on below link:
https://www.cdc.gov/hepatitis/hav/guidelinesa.htm
CONSULTATION AND LONG TERM MONITORING
Encourage patients to have an adequate diet. Patients should avoid alcohol and medications that may accumulate in liver disease. Otherwise, no specific dietary restrictions are necessary.
Bed rest during the acute illness may be important, although data to support this practice are lacking. Restricting transmission is important, especially in the early phases of the illness. Returning to work should probably be delayed for 10 days after the onset of jaundice.
PRECAUTIONS
Protection Prior to Exposure
Tools for prevention of HAV infection include vaccination, immune globulin, and attention to hygienic practices.
Indications: Advisory Committee on Immunization Practices of the United States Centers for Disease Control and Prevention recommends protection (ideally vaccination) prior to potential hepatitis A exposure for the following individuals:(44-48)
- All children at age one year (i.e. 12 to 23 months); children who have not been vaccinated by age two can be vaccinated at subsequent visits.
- Children between ages 2 and 18 years who live in regions where routine hepatitis A vaccination has been implemented because of high disease incidence.
- Individuals traveling to or working in countries with high or intermediate rates of HAV
- Men who have sex with men.
- Illicit drug users (injection and noninjection).
- Individuals working with HAV-infected primates or with HAV in a research laboratory.
- Individuals with chronic liver disease. Patients with chronic hepatitis B and C virus are at risk for HAV superinfection and fulminant hepatic failure.(49,50) Immunization against HAV infection appears to be safe and effective in patients with chronic liver disease, including hepatitis C virus infection.(51-55)
- Individuals with clotting factor disorders.
- Individuals with direct contact to others who have hepatitis A.
- Homeless individuals.
Clinical approach: The primary tool for protection against hepatitis A prior to exposure is vaccination, which is superior to immune globulin with respect to achievable antibody concentrations and durability of immune response.(56,57) For individuals at risk for hepatitis A exposure who are allergic to the hepatitis A vaccine or are <12 months of age, passive immunization via immune globulin may be given.
Vaccination
Available vaccines: Hepatitis A vaccine is a suspension for injection in a pre-filled syringe (inactivated, adsorbed) is available in Pakistan.
Passive immunization: Immune globulin can decrease the incidence of HAV infection by more than 90 percent.(58-61)
Groups warranting immune globulin in addition to HAV vaccination (administered at a separate anatomic site) include:
- Adult travelers >40 years, based on the provider’s risk assessment.
- Individuals with chronic liver disease.
- Immunocompromised individuals incapable of mounting an adequate immune response to HAV vaccine.
Groups warranting immune globulin (in the absence of HAV vaccination) include:
- Infant travelers <6 months of age.
- Travelers for whom vaccine is contraindicated (e.g. who are allergic to the hepatitis A vaccine).
Protection Following Exposure
Indications: Individuals who warrant postexposure protection (i.e. hepatitis A vaccine and/or immune globulin) after exposure to HAV include:(62)
- Close personal contacts of an individual with laboratory-confirmed HAV infection:
- Household and sexual contacts
- Individuals who have shared illicit drugs
- Child care center contacts, in the setting of ≥1 case of hepatitis A among children or staff or ≥2 household cases of center attendees:
- For centers providing care to children in diapers – Postexposure protection is warranted for all previously unvaccinated staff and attendees. In the setting of an outbreak (cases in ≥3 families), postexposure protection is also appropriate for household members of diaper-wearing children.
- For centers providing care to children who no longer wear diapers – Postexposure protection is warranted for classroom contacts of the index patient (but not for children or staff in other classrooms).
- Food handlers:
- In establishments with a food handler diagnosed with hepatitis A, postexposure protection is warranted for other food handlers at the same establishment. Administration of postexposure protection to patrons is typically is not indicated; it is appropriate if the food handler had diarrhea or poor hygienic practices and directly handled uncooked foods or foods following cooking, and patrons can be identified and prophylaxed within two weeks of exposure.
- Postexposure prophylaxis is reasonable in settings in which repeated exposures to hepatitis A might have occurred, such as institutional cafeterias.
Hygienic Practices: Hygienic practices for prevention of HAV infection include:(41,63)
- Handwashing (including after using the bathroom, changing diapers, and before preparing or eating foods).
- Avoiding tap water and raw foods in areas with poor sanitation.
- Heating foods appropriately (the virus can be inactivated by heating to >185°F [>85°C] for one minute). Cooked foods can transmit HAV if the temperature during food preparation is inadequate to kill the virus or if food is contaminated after cooking.
Chlorine, iodine, and disinfecting solutions (household bleach 1:100 dilution) are effective for inactivation of HAV.
REFERENCES
- Ansaldi F, Bruzzone B, Rota MC, et al. Hepatitis A incidence and hospital-based seroprevalence in Italy: a nation-wide study. Eur J Epidemiol. 2008. 23(1):45-53.
- Domínguez A, Bruguera M, Plans P, et al. Declining hepatitis A seroprevalence in adults in Catalonia (Spain): a population-based study. BMC Infect Dis. 2007. 7:73.
- Aggarwal R, Goel A. Hepatitis A: epidemiology in resource-poor countries. Curr Opin Infect Dis. 2015 Oct. 28 (5):488-96.
- World Health Organization. Global Alert and Response (GAR): Hepatitis A.
http://www.who.int/csr/disease/hepatitis/whocdscsredc2007/en/index4.html#estimated (Accessed on Nov 14, 2018). - Wasley A, Grytdal S, Gallagher K,. Surveillance for acute viral hepatitis–United States, 2006. MMWR Surveill Summ. 2008 Mar 21. 57(2):1-24.
- Chobe LP, Arankalle VA. Investigation of a hepatitis A outbreak from Shimla Himachal Pradesh. Indian J Med Res. 2009 Aug. 130(2):179-84.
- Cao J, Wang Y, Song H, Meng Q, Sheng L, Bian T, et al. Hepatitis A outbreaks in China during 2006: application of molecular epidemiology. Hepatol Int. 2009 Jun. 3(2):356-63.
- Kamath SR, Sathiyasekaran M, Raja TE, Sudha L. Profile of viral hepatitis A in Chennai. Indian Pediatr. 2009 Jul. 46(7):642-3.
- Fischer GE, Thompson N, Chaves SS, Bower W, Goldstein S, Armstrong G, et al. The epidemiology of hepatitis A virus infections in four Pacific Island nations, 1995-2008. Trans R Soc Trop Med Hyg. 2009 Sep. 103(9):906-10.
- Amin J, Gilbert GL, Escott RG, et al. Hepatitis A epidemiology in Australia: national seroprevalence and notifications. Med J Aust. 2001 Apr 2. 174(7):338-41.
- Longatti A. The dual role of exosomes in hepatitis A and C virus transmission and viral immune activation. Viruses. 2015 Dec 17. 7 (12):6707-15.
- Kaplan G, Totsuka A, Thompson P et al. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 1996 Aug 15. 15(16):4282-96.
- Richard K Gilroy. Hepatitis A Clinical Presentation. Medscape. WebMD LLC. https://emedicine.medscape.com/article/177484-clinical (Accessed on Nov 15, 2018).
- Lemon SM. Type A viral hepatitis. New developments in an old disease. N Engl J Med 1985; 313:1059
- Lednar WM, Lemon SM, Kirkpatrick JW, et al. Frequency of illness associated with epidemic hepatitis A virus infections in adults. Am J Epidemiol 1985; 122:226
- Diagnosis and management of foodborne illnesses: a primer for physicians and other health care professionals. MMWR Recomm Rep 2004; 53(RR-4):1.
- Michelle Lai, Sanjiv Chopra. Hepatitis A virus infection in adults: Epidemiology, clinical manifestations, and diagnosis. Uptodate. Wolters Kluwer.
https://www.uptodate.com/contents/hepatitis-a-virus-infection-in-adults-epidemiology-clinical-manifestations-and-diagnosis?
search=hepatitis%20A&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (Accessed on Nov 15, 2018). - Cuthbert JA. Hepatitis A: old and new. Clin Microbiol Rev 2001; 14:38.
- Tong MJ, el-Farra NS, Grew MI. Clinical manifestations of hepatitis A: recent experience in a community teaching hospital. J Infect Dis 1995; 171 Suppl 1:S15.
- Koff RS. Clinical manifestations and diagnosis of hepatitis A virus infection. Vaccine 1992; 10 Suppl 1:S15
- Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J 2001; 20:380
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1
- Kemmer NM, Miskovsky EP. Hepatitis A. Infect Dis Clin North Am 2000; 14:605
- Inman RD, Hodge M, Johnston ME, et al. Arthritis, vasculitis, and cryoglobulinemia associated with relapsing hepatitis A virus infection. Ann Intern Med 1986; 105:700
- Dan M, Yaniv R. Cholestatic hepatitis, cutaneous vasculitis, and vascular deposits of immunoglobulin M and complement associated with hepatitis A virus infection. Am J Med 1990; 89:103
- Schiff ER. Atypical clinical manifestations of hepatitis A. Vaccine 1992; 10 Suppl 1:S18
- Ilan Y, Hillman M, Oren R, et al. Vasculitis and cryoglobulinemia associated with persisting cholestatic hepatitis A virus infection. Am J Gastroenterol 1990; 85:586
- Lavine J, Bull F, Millward-Sadler G. Acute viral hepatitis. In: Wright’s Liver and Biliary Disease, Millard-Sadler G, Wright R, Arthur M (Eds), WB Saunders, London 1992. p.681
- Shenoy R, Nair S, Kamath N. Thrombocytopenia in hepatitis A–an atypical presentation. J Trop Pediatr 2004; 50:241
- Gordon SC, Reddy KR, Schiff L, Schiff ER. Prolonged intrahepatic cholestasis secondary to acute hepatitis A. Ann Intern Med 1984; 101:635
- Jung YM, Park SJ, Kim JS, et al. Atypical manifestations of hepatitis A infection: a prospective, multicenter study in Korea. J Med Virol 2010; 82:1318
- Schiraldi O, Modugno A, Miglietta A, Fera G. Prolonged viral hepatitis type A with cholestasis: case report. Ital J Gastroenterol 1991; 23:364
- Glikson M, Galun E, Oren R, et al. Relapsing hepatitis A. Review of 14 cases and literature survey. Medicine (Baltimore) 1992; 71:14
- Kassas AL, Telegdy L, Méhesfalvi E, et al. Polyphasic and protracted patterns of hepatitis A infection: a retrospective study. Acta Med Hung 1994; 50:93
- Bornstein JD, Byrd DE, Trotter JF. Relapsing hepatitis A: a case report and review of the literature. J Clin Gastroenterol 1999; 28:355.
- Grünhage F, Spengler U, Fischer HP, Sauerbruch T. Autoimmune hepatitis–sequel of a relapsing hepatitis A in a 75-year-old woman. Digestion 2004; 70:187
- Sjogren MH, Tanno H, Fay O, et al. Hepatitis A virus in stool during clinical relapse. Ann Intern Med 1987; 106:221
- Rachima CM, Cohen E, Garty M. Acute hepatitis A: combination of the relapsing and the cholestatic forms, two rare variants. Am J Med Sci 2000; 319:417
- Vento S, Garofano T, Di Perri G, et al. Identification of hepatitis A virus as a trigger for autoimmune chronic hepatitis type 1 in susceptible individuals. Lancet 1991; 337:1183
- Skoog SM, Rivard RE, Batts KP, Smith CI. Autoimmune hepatitis preceded by acute hepatitis A infection. Am J Gastroenterol 2002; 97:1568
- Centers for Disease Control and Prevention. Hepatitis A Questions and Answers for Health Professionals. http://www.cdc.gov/hepatitis/hav/havfaq.htm#general (Accessed on November 16, 2018).
- Kodani M, Mixson-Hayden T, Drobeniuc J, Kamili S. Rapid and sensitive approach to simultaneous detection of genomes of hepatitis A, B, C, D and E viruses. J Clin Virol. 2014 Oct. 61 (2):260-4.
- Kanda T, Nakamoto S, Wu S, et al. Direct-acting antivirals and host-targeting agents against the hepatitis A virus. J Clin Transl Hepatol. 2015 Sep 28. 3 (3):205-10.
- Nelson NP, Link-Gelles R, Hofmeister MG, et al. Update: Recommendations of the Advisory Committee on Immunization Practices for Use of Hepatitis A Vaccine for Postexposure Prophylaxis and for Preexposure Prophylaxis for International Travel. MMWR Morb Mortal Wkly Rep 2018; 67:1216
- Foster M, Ramachandran S, Myatt K, et al. Hepatitis A Virus Outbreaks Associated with Drug Use and Homelessness – California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep 2018; 67:1208
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1080
- Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices. Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morb Mortal Wkly Rep 2009; 58:1006
- American Academy of Pediatrics Committee on Infectious Diseases. Recommendations for administering hepatitis A vaccine to contacts of international adoptees. Pediatrics 2011; 128:803.
- Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1999; 48:1
- Vento S, Garofano T, Renzini C, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med 1998; 338:286
- Myers RP, Gregor JC, Marotta PJ. The cost-effectiveness of hepatitis A vaccination in patients with chronic hepatitis C. Hepatology 2000; 31:834
- Jacobs RJ, Koff RS, Meyerhoff AS. The cost-effectiveness of vaccinating chronic hepatitis C patients against hepatitis A. Am J Gastroenterol 2002; 97:427
- Arguedas MR, Heudebert GR, Fallon MB, Stinnett AA. The cost-effectiveness of hepatitis A vaccination in patients with chronic hepatitis C viral infection in the
United States. Am J Gastroenterol 2002; 97:721 - Keeffe EB, Iwarson S, McMahon BJ, et al. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology 1998; 27:881
- Dumot JA, Barnes DS, Younossi Z, et al. Immunogenicity of hepatitis A vaccine in decompensated liver disease. Am J Gastroenterol 1999; 94:1601
- Leentvaar-Kuijpers A, Coutinho RA, Brulein V, Safary A. Simultaneous passive and active immunization against hepatitis A. Vaccine 1992; 10 Suppl 1:S138
- Shouval D, Ashur Y, Adler R, et al. Safety, tolerability, and immunogenicity of an inactivated hepatitis A vaccine: effects of single and booster injections, and comparison to administration of immune globulin. J Hepatol 1993; 18 Suppl 2:S32
- Stokes J, Neefe JR. The prevention and attenuation of infectious hepatitis by gamma globulin: Preliminary note. JAMA 1945; 127:144
- Winokur PL, Stapleton JT. Immunoglobulin prophylaxis for hepatitis A. Clin Infect Dis 1992; 14:580
- Conrad ME, Lemon SM. Prevention of endemic icteric viral hepatitis by administration of immune serum gamma globulin. J Infect Dis 1987; 156:56
- Stapleton JT. Passive immunization against hepatitis A. Vaccine 1992; 10 Suppl 1:S45
- Centers for Disease Control and Prevention. Hepatitis A Questions and Answers for Health Professionals. http://www.cdc.gov/hepatitis/hav/havfaq.htm#vaccine (Accessed on November 16, 2018).
- Mbithi JN, Springthorpe VS, Boulet JR, Sattar SA. Survival of hepatitis A virus on human hands and its transfer on contact with animate and inanimate surfaces. J Clin Microbiol 1992; 30:757
