EPIDEMIOLOGY
Available data suggest that approximately 15 to 20 million of the 257 million HBV carriers worldwide may be infected with HDV.(1,2)
The prevalence of HDV infection among HBV carriers in Asia is quite variable. Although low in Japan,(3) other countries, especially Mongolia and those in Central Asia, are heavily affected, and the prevalence among HBV carriers can be as high as 60 percent in some areas in Pakistan.(4)
There are three known genotypes of HDV. Genotype I has a worldwide distribution; genotype 2 exists in Taiwan, Japan, and northern Asia; and genotype 3 is found in South America.(5)
PATHOPHYSIOLOGY
The detailed mechanisms by which HDV induces liver damage are unknown. However, the pathogenesis of hepatitis D-related liver disease appears to depend on the interplay of three major factors:
- HDV-associated factors, such as genotype(6) and the expression of specific HDAg species.(7)
- Host-associated factors, such as the immune response
- Helper virus-associated factors, such as the HBV genotype and the level of HBV replication.(8)
HDV Infection:
Due to its dependence upon HBV, HDV infection always occurs in association with HBV infection.
Coinfection: Coinfection of HBV and HDV in an individual susceptible to HBV infection (i.e. anti-HBs-negative) results in acute hepatitis B + D. This entity is clinically indistinguishable from classical acute hepatitis B and is usually transient and self-limited. However, a high incidence of liver failure has been reported among injection drug users.(9)
The rate of progression to chronic infection is not different from that observed after classical acute hepatitis B, since persistence of HDV infection is dependent upon persistence of HBV infection.(10)
Superinfection: HDV superinfection of a chronic HBsAg carrier may present as a severe acute hepatitis in a previously unrecognized HBV carrier, or as an exacerbation of preexisting chronic hepatitis B. Progression to chronic HDV infection occurs in almost all patients.(11) However, HBV replication is usually suppressed by HDV.
TRANSMISSION
HDV is transmitted parenterally; it can replicate independently within the hepatocyte, but it requires hepatitis B surface antigen (HBsAg) for propagation.
Risk factors include:
- Intravenous drug use and multiple blood transfusions.
- Sexual transmission is less efficient than with hepatitis B virus (HBV).
- Perinatal transmission is rare.
NATURAL HISTORY
Genotype 1: In the Western world, where the predominant genotype is genotype 1,(14) acute hepatitis D has an increased risk of acute liver failure when compared with acute hepatitis B.(9) Once chronic HDV infection is established, it usually exacerbates the preexisting liver disease due to HBV.(11) Progression towards cirrhosis may be rapid.(15,16)
Genotype 2: In the Far East, where the predominant genotype is genotype 2, there is a reduced risk of acute liver failure and rapidly progressive liver disease.(13,17)
Genotype 3: Severe outbreaks of acute hepatitis D with a high incidence of acute liver failure have been reported among the Yukpa Indians of Venezuela,(18) the Sierra Nevada de Santa Marta in Colombia,(19) and some remote areas of the Brazilian(20) and Peruvian(6) Amazon basin. Viral factors have been postulated to be related to the fulminant course in these outbreaks, as HDV isolates from Colombia and Peru belong to a distinct viral genotype denoted genotype 3.(6)
Other genotypes: At least five additional HDV genotypes have been described.(21,22) Sequences previously assigned to genotype 2b are now classified as genotype 4, and African sequences seem to cluster into four additional genotypes, named from 5 to 8. These new genotypes are less well characterized as to their disease features compared with genotypes 1 to 3.
SIGN AND SYMPTOMS
Hepatitis D virus (HDV) infection is clinically indistinguishable from other forms of viral hepatitis. As many as 90% of patients are asymptomatic.
The incubation period is 21-45 days but may be shorter in cases of superinfection.
Signs/symptoms include the following:
- Jaundice
- Dark urine
- Abdominal pain
- Nausea with vomiting
- Confusion, bruising, and bleeding (rare)
- Pruritus
Signs/symptoms upon presentation include the following:
- Scleral icterus
- Fever
- Abdominal pain, usually right upper quadrant
- Tea-colored urine
- Encephalopathy (rare)
- Petechia with bruising (rare)
SCREENING
Although screening for hepatitis delta virus (HDV) may be beneficial in patients with hepatitis B virus (HBV), there are currently no consensus statements (for example, from the CDC or the American Association for the Study of Liver Diseases [AASLD]) that recommend general screening.
DIAGNOSTIC TESTS
The HD virion is composed of an outer lipoprotein envelope made of the surface antigen of the HBV (HBsAg) and an inner ribonucleoprotein structure in which the HDV genome resides. The HDV genome consists of a single stranded RNA which is folded as a rod-like structure through internal base-pairing. It is complexed with the only HDV-encoded antigen, the HDAg.(23)
HDAg can elicit a specific immune response in the infected host, consisting of antibodies of the IgM and IgG class (anti-HDV). In HDV infected individuals, the timing of appearance and level of HDV RNA, HDAg, and anti-HDV in serum allow the three HDV-related clinical entities to be discriminated:
- Acute HBV/HDV coinfection
- Acute HDV superinfection of a chronic HBV carrier
- Chronic HDV infection
Due to the dependence of HDV on HBV, the presence of HBsAg is necessary for the diagnosis of HDV infection. The additional presence of IgM antibody to hepatitis B core antigen (IgM anti-HBc) is necessary for the diagnosis of acute HBV/HDV coinfection (below table).
Diagnosis of hepatitis D virus infection in different clinical settings
HBsAg
Anti-HBc, IgM
Serum HDAg (by EIA/RIA)
Serum HDV RNA (by hybridization)
Anti-HDV, total
Anti-HDV, IgM
Liver HDAg
Positive
Positive
Early and short-lived, frequently missed
Early, transient but last longer than HDAg
Late, low titer
Transient, may be the only marker
Not indicated
Positive
Negative
Early and transient, and frequently missed
Early and persistent
Rapidly increasing titers
Rapidly increasing and persistent titers
Positive
Positive
Negative
Early and transient, and frequently missed
Early and persistent
Rapidly increasing titers
Rapidly increasing and persistent titers
Positive
Detection of serum HDAg
Serum HDAg can be detected by microplate-based, enzyme-linked (EIA) or radioimmunoassays (RIA). At present, these assays are not available for clinical diagnosis in the majority of countries.
- In acute HDV infection, serum HDAg appears early but is very short-lived and may escape detection if repeated testing is not performed.(24-27) HDAg lasts longer when the immune response is slow and weak as in immunodeficient individuals.(28)
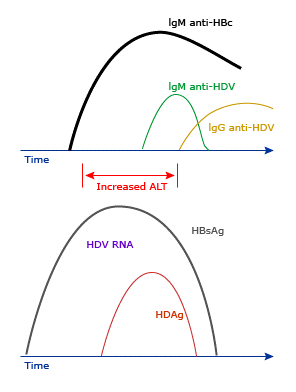
Schematic representation of the typical serologic response to acute HBV/HDV infection. Patients should be positive for HBsAg and have high titer IgM anti-HBc; serum HDAg and/or HDV RNA are usually positive at presentation. (courtesy Uptodate)
- In chronic HDV infection, anti-HDV is present in high titers. Thus, HDAg cannot be detected by microwell assays since it is complexed with anti-HDV. Serum HDAg is best detected in this setting by immunoblot assay,(29) since polyacrylamide gel electrophoresis under denaturing conditions allows separation of HDAg from anti-HDV. Immunoblot assay is very sensitive(30) but technically difficult, time- and labor-consuming, and cannot be used for routine detection of HDAg.
Detection of serum HDV RNA
HDV RNA can be detected in serum by reverse transcriptase-polymerase chain reaction (RT-PCR)-based assays.
RT-PCR assays have a detection limit of less than 10 genomes per mL.(31-35) However, the extensive sequence heterogeneity of different HDV isolates makes it difficult to choose suitable primers for the amplification of HDV RNA since only a few conserved regions exist in the HDV genome. Furthermore, the secondary and tertiary structure of the HDV RNA may hamper efficient amplification even of highly conserved regions.(34)
An interesting application of the RT-PCR assay for serum HDV RNA is the analysis of the different HDV genotypes. Genotyping can be performed by direct sequencing or restriction fragment length polymorphism of the amplicon.(6,36-38) Since the clinical relevance of HDV genotyping is uncertain, its widespread use is at present not recommended.
Detection of anti-HDV antibody:
Total (IgM and IgG) anti-HDV antibodies can be detected by EIAs or RIAs.
- Total anti-HDV antibody appears usually after four weeks of acute infection in acute hepatitis D. As a result, its clinical value is limited unless repeated testing is performed.(24,27) Nevertheless, a well-documented anti-HDV seroconversion may be the only way to diagnose acute HDV infection in the absence of other markers of HDV infection.
- High-titer anti-HDV of the IgG class is present in chronic HDV infection. It correlates well with ongoing HDV replication and may help in differentiating current from past HDV infection.(39)
- IgM anti-HDV is transient and delayed if the course of acute hepatitis D is self-limited, but it may be the only serum marker of acute HDV infection.(24) In patients who progress to chronic HDV infection, which is usually the case in those with HDV superinfection, IgM anti-HDV is brisk and long-lasting. It should be remembered, however, that differentiating between HBV/HDV coinfection and HDV superinfection in an HBV carrier relies mainly on the detection of high-titer IgM anti-HBc in patients with coinfection.
IgM anti-HDV is present in high titers during chronic HDV infection, and the titers correlate with the level of HDV replication and severity of liver disease.(40) IgM anti-HDV gradually disappears from serum in patients who have persistent remission after interferon therapy and following liver transplantation.(41)
Tissue markers of HDV infection:
Both HDAg and HDV RNA can be detected in liver tissues routinely processed for histopathologic evaluation.
- HDAg can be detected by direct immunofluorescence or immunohistochemical staining. The detection of intrahepatic HDAg has been proposed as the “gold” standard for the diagnosis of current HDV infection.(42) However, as many as 50 percent of liver biopsy specimens from patients who have been infected for 10 or more years may be negative for HDAg, suggesting that the levels of HDV replication may decrease with time.(17,43) In patients who are negative for HDAg, the diagnosis of current HDV infection has to rely on the detection of HDV RNA and high-titer anti-HDV antibodies in the serum.
- HDV RNA can be detected by in-situ hybridization. However, the techniques involved are too time-consuming, tedious, and difficult for clinical use.(44)
Imaging studies
Right upper quadrant ultrasonography helps in the evaluation for biliary obstruction and hepatocellular carcinoma.
Perform cholescintigraphy (hydroxy iminodiacetic acid) to exclude acute cholecystitis, if clinically indicated.
Perform computed tomography (CT) scanning or magnetic resonance imaging (MRI) if hepatocellular carcinoma is suggested. (An alpha-fetoprotein (AFP) level greater than 250 ng/mL is highly suggestive of hepatocellular carcinoma (HCC).)
PATIENT SELECTION FOR TREATMENT
For patients with chronic hepatitis D virus (HDV) infection, treatment for those with elevated HDV RNA levels and active liver disease (as evidenced by elevated serum aminotransferase [ALT] levels and/or chronic hepatitis on liver biopsy) is suggested. Patients should be treated early, particularly if there is advanced fibrosis. Asymptomatic HDV carriers with persistently normal ALT levels do not require therapy but should be monitored for signs of active disease (e.g. every six months).
Eradication of HBV infection with development of hepatitis B surface antibody (anti-HBs) will protect the individual from reinfection with HBV as well as HDV. Patients who have cleared HDV but who remain hepatitis B surface antigen (HBsAg) positive are still at risk of reinfection with HDV.
THERAPY CONSIDERATION
Treatment for infection with hepatitis D virus (HDV) consists primarily of supportive measures. Observe synthetic liver function markers and mental status closely. Deterioration of either should prompt early consultation with hospital personnel capable of performing liver transplantation.
Liver transplantation is indicated in patients with fulminant liver failure. Patients with evidence of decompensated liver disease or fulminant liver failure should be immediately transferred to a center capable of performing a liver transplantation.
No vaccine is available for HDV, but the hepatitis B virus (HBV) vaccination is effective against HDV.
TREATMENT OPTIONS
For those who require therapy, pegylated interferon alfa (IFNa) is generally the treatment of choice and should be administered for one year.(45) Either pegylated IFN alfa-2a (180 mcg weekly) or pegylated IFN alfa-2b (1.5 mcg/kg weekly) can be used. However, the optimal treatment for HDV is uncertain, and patients can be referred to specialized centers that offer experimental therapies.
There is no benefit of adding a nucleos(t)ide analogue for the treatment of HDV. However, tenofovir or entecavir should be added if treatment for hepatitis B virus (HBV) is warranted to achieve maximal suppression of both viruses.
Interferon alfa:
The only available drug effective against HDV is interferon alfa (IFNa). Peginterferon appears to be more effective than standard interferon, but data are limited. Unfortunately, only a minority of patients treated with interferon clear HDV infection. A meta-analysis of five trials comparing interferon with observation (including a total of 169 participants) concluded that there was a modest benefit in suppressing viral and liver disease activity in some patients, but such benefits were not sustained in the majority of patients.(46)
The mechanism of action of IFNa in hepatitis D is unclear. IFNa does not have any antiviral activity against HDV when tested in vitro.(47,48) Thus, the efficacy of IFNa in patients with chronic hepatitis D may depend upon its antiviral effects on the helper virus (i.e. HBV) or its immunomodulatory effects.
Experimental Treatments:
Several drugs have been evaluated as alternatives to interferon (e.g. ribavirin, foscarnet, acyclovir). Many of these agents were used to treat other infections and were thought to have activity against hepatitis D virus (HDV) based upon in vitro studies or small case reports. However, results in subsequent human studies were discouraging.(49-59)
Newer agents that have novel mechanisms of action show more promise. As examples:
- Specific inhibitors of HDV prenylation: “Prenylation” involves the covalent addition of a farnesyl or geranylgeranyl isoprenoid molecule to a conserved cysteine residue at or near the C-terminus of a protein.(60) This link promotes membrane interactions with the prenylated protein since the isoprenoid chain is hydrophobic.
Lonafarnib, a farnesyltransferase inhibitor used to treat other diseases (e.g. progeria), was evaluated in a phase 2a study of 14 patients with HDV.(61) Eight patients received 100 mg of lonafarnib twice daily, and six patients received 200 mg of lonafarnib twice daily. Treatment was administered for 28 days, and when compared with placebo, resulted in a greater decline in HDV RNA (average serum HDV RNA decline of 0.73 log and 1.54 log international units/mL for the lower and higher doses, respectively).
- HDV entry inhibitors: These agents act upon the sodium taurocholate cotransporting polypeptide (NTCP), which is a receptor shared by HBV and HDV .(62,63) Example: Myrcludex B
Inhibitors of virion secretion: REP 2139 is a nucleic acid polymer that has been shown to clear HBsAg by blocking the release of subviral particles. This agent was evaluated for the treatment of HDV infection in an uncontrolled phase 2 study.(64,65)
GOAL OF THERAPY
The primary goal of treatment is suppression of HDV RNA 24 weeks after completing treatment, accompanied by normalization of the ALT level. Although the rate of virologic suppression with IFN is low, a response is more likely to be attained in patients with a shorter duration of infection. Successful treatment is associated with amelioration of necroinflammatory activity and loss of hepatitis D antigen (HDAg) in the liver.
GUIDELINES
To view, Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance, please click on below email.
https://www.aasld.org/sites/default/files/HBVGuidance_Terrault_et_al-2018-Hepatology.pdf
To view, EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection, please click on below link:
http://www.easl.eu/medias/cpg/management-of-hepatitis-B-virus-infection/English-report.pdf
To view, WHO hepatitis D guidance document, please click on below link:
http://www.who.int/news-room/fact-sheets/detail/hepatitis-d
To view, Diagnosis, management and treatment of hepatitis delta virus infection: Turkey 2017 Clinical Practice Guidelines, please click on below link:
http://www.turkjgastroenterol.org/sayilar/304/buyuk/S84-S89.pdf
CONSULTATION AND LONG TERM MONITORING
Follow-up is recommended for at least 6 months to determine if chronic hepatitis B virus (HBV) and hepatitis D virus (HDV) infection develop. Perform a liver biopsy to stage liver disease prior to beginning interferon alfa therapy. Treatment with interferon can be continued after the 1-year period if well tolerated and efficacy is demonstrated. Monitoring HDV RNA and hepatitis B surface antigen (HBsAg) levels may help in guiding therapy.
Early notification of a hepatologist or gastroenterologist is warranted. Diet need not be restricted. If enteral intake is poor, intravenous fluids can be administered. Total parental nutrition is seldom needed.
PRECAUTIONS
- For hepatitis B virus (HBV) carriers, every effort should be made to reduce the risk of hepatitis D virus (HDV) transmission.
- For others, the mainstay of prevention of HDV infection is vaccination against HBV. Partial protection of hepatitis B surface antigen (HBsAg) carriers from HDV infection through active immunization may be feasible. However, worldwide implementation of a vaccination policy against HDV would probably prove too costly and impractical due to the need to screen for HBsAg carriers. Vaccination against HBV remains the most cost-effective means to prevent HDV infection, except for individuals who are already infected with HBV.
- Educate patients regarding modification of high-risk behaviors, including intravenous drug use and unsafe sexual practices.
- Promote the use of universal precautions for health care workers.
- Discuss with patients with chronic HDV and HBV infection that they should not donate blood, share toothbrushes or razors, or consume alcohol. Precautions should be observed regarding blood and body fluids.
REFERENCES
- Wedemeyer H, Manns MP. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol 2010; 7:31.
- World Health Organization. Global Hepatitis Report, 2017. http://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf;jsessionid=21B5AA5BEFA9E117AF03AAEAB7EECAD1?sequence=1 (Accessed on November 27, 2018).
- Rizzetto M, Ponzetto A, Forzani I. Epidemiology of hepatitis delta virus: overview. Prog Clin Biol Res 1991; 364:1.
- Abbas Z, Jafri W, Raza S. Hepatitis D: Scenario in the Asia-Pacific region. World J Gastroenterol 2010; 16:554.
- Praveen K Roy. Hepatitis D. Medscape. https://emedicine.medscape.com/article/178038-overview (Accessed on November 27, 2018)
- Casey JL, Brown TL, Colan EJ, et al. A genotype of hepatitis D virus that occurs in northern South America. Proc Natl Acad Sci U S A 1993; 90:9016.
- Tang JR, Hantz O, Vitvitski L, et al. Discovery of a novel point mutation changing the HDAg expression of a hepatitis delta virus isolate from Central African Republic. J Gen Virol 1993; 74 ( Pt 9):1827.
- Smedile A, Rosina F, Saracco G, et al. Hepatitis B virus replication modulates pathogenesis of hepatitis D virus in chronic hepatitis D. Hepatology 1991; 13:413.
- Smedile A, Farci P, Verme G, et al. Influence of delta infection on severity of hepatitis B. Lancet 1982; 2:945.
- Caredda F, d’Arminio Monforte A, Rossi E, et al. Prospective study of epidemic delta infection in drug addicts. Prog Clin Biol Res 1983; 143:245
- Smedile A, Dentico P, Zanetti A, et al. Infection with the delta agent in chronic HBsAg carriers. Gastroenterology 1981; 81:992
- Romeo R, Del Ninno E, Rumi M, et al. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 2009; 136:1629.
- Wu JC, Choo KB, Chen CM, et al. Genotyping of hepatitis D virus by restriction-fragment length polymorphism and relation to outcome of hepatitis D. Lancet 1995; 346:939
- Niro GA, Smedile A, Andriulli A, et al. The predominance of hepatitis delta virus genotype I among chronically infected Italian patients. Hepatology 1997; 25:728
- Rizzetto M, Verme G, Recchia S, et al. Chronic hepatitis in carriers of hepatitis B surface antigen, with intrahepatic expression of the delta antigen. An active and progressive disease unresponsive to immunosuppressive treatment. Ann Intern Med 1983; 98:437
- Govindarajan S, De Cock KM, Redeker AG. Natural course of delta superinfection in chronic hepatitis B virus-infected patients: histopathologic study with multiple liver biopsies. Hepatology 1986; 6:640
- Wu JC, Chen TZ, Huang YS, et al. Natural history of hepatitis D viral superinfection: significance of viremia detected by polymerase chain reaction. Gastroenterology 1995; 108:796
- Hadler SC, Alcala de Monzon M, Rivero D, et al. Epidemiology and long-term consequences of hepatitis delta virus infection in the Yucpa Indians of Venezuela. Am J Epidemiol 1992; 136:1507
- Popper H, Thung SN, Gerber MA, et al. Histologic studies of severe delta agent infection in Venezuelan Indians. Hepatology 1983; 3:906
- Bensabath G, Hadler SC, Soares MC, et al. Hepatitis delta virus infection and Labrea hepatitis. Prevalence and role in fulminant hepatitis in the Amazon Basin. JAMA 1987; 258:479
- Dény P. Hepatitis delta virus genetic variability: from genotypes I, II, III to eight major clades? Curr Top Microbiol Immunol 2006; 307:151
- Le Gal F, Gault E, Ripault MP, et al. Eighth major clade for hepatitis delta virus. Emerg Infect Dis 2006; 12:1447
- Taylor J, Negro F, Rizzetto M. Hepatitis delta virus: From structure to disease expression. Rev Med Virol 1992; 2:161
- Aragona M, Macagno S, Caredda F, et al. Serological response to the hepatitis delta virus in hepatitis D. Lancet 1987; 1:478
- Govindarajan S, Valinluck B, Peters L. Relapse of acute B viral hepatitis–role of delta agent. Gut 1986; 27:19
- Shattock AG, Morgan BM. Sensitive enzyme immunoassay for the detection of delta antigen and anti-delta, using serum as the delta antigen source. J Med Virol 1984; 13:73
- Buti M, Esteban R, Jardí R, et al. Serological diagnosis of acute delta hepatitis. J Med Virol 1986; 18:81
- Grippon P, Ribiere O, Cadranel JF, et al. Long-term delta antigenaemia without appearance of delta antibody in two immunodeficient patients. Lancet 1987; 1:1031
- Bonino F, Heermann KH, Rizzetto M, Gerlich WH. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J Virol 1986; 58:945
- Buti M, Esteban R, Jardi R, et al. Chronic delta hepatitis: detection of hepatitis delta virus antigen in serum by immunoblot and correlation with other markers of delta viral replication. Hepatology 1989; 10:907
- Zignego AL, Deny P, Feray C, et al. Amplification of hepatitis delta virus RNA sequences by polymerase chain reaction: a tool for viral detection and cloning. Mol Cell Probes 1990; 4:43.
- Madejón A, Castillo I, Bartolomé J, et al. Detection of HDV-RNA by PCR in serum of patients with chronic HDV infection. J Hepatol 1990; 11:381.
- Cariani E, Ravaggi A, Puoti M, et al. Evaluation of hepatitis delta virus RNA levels during interferon therapy by analysis of polymerase chain reaction products with a nonradioisotopic hybridization assay. Hepatology 1992; 15:685.
- Dinolfo L, Abate ML, Bertolo P, et al. Detection of hepatitis D virus RNA in serum by a reverse transcription, polymerase chain reaction-based assay. Int J Clin Lab Res 1995; 25:35.
- Wu JC, Chen TZ, Huang YS, et al. Natural history of hepatitis D viral superinfection: significance of viremia detected by polymerase chain reaction. Gastroenterology 1995; 108:796.
- Wu JC, Choo KB, Chen CM, et al. Genotyping of hepatitis D virus by restriction-fragment length polymorphism and relation to outcome of hepatitis D. Lancet 1995; 346:939.
- Casey JL, Niro GA, Engle RE, et al. Hepatitis B virus (HBV)/hepatitis D virus (HDV) coinfection in outbreaks of acute hepatitis in the Peruvian Amazon basin: the roles of HDV genotype III and HBV genotype F. J Infect Dis 1996; 174:920.
- Niro GA, Smedile A, Andriulli A, et al. The predominance of hepatitis delta virus genotype I among chronically infected Italian patients. Hepatology 1997; 25:728.
- Negro F, Bergmann KF, Baroudy BM, et al. Chronic hepatitis D virus (HDV) infection in hepatitis B virus carrier chimpanzees experimentally superinfected with HDV. J Infect Dis 1988; 158:151
- Smedile A, Lavarini C, Crivelli O, et al. Radioimmunoassay detection of IgM antibodies to the HBV-associated delta (delta) antigen:” clinical significance in delta infection. J Med Virol 1982; 9:131
- Borghesio E, Rosina F, Smedile A, et al. Serum immunoglobulin M antibody to hepatitis D as a surrogate marker of hepatitis D in interferon-treated patients and in patients who underwent liver transplantation. Hepatology 1998; 27:873
- Di Bisceglie AM, Negro F. Diagnosis of hepatitis delta virus infection. Hepatology 1989; 10:1014
- Bonino F, Negro F, Baldi M, et al. The natural history of chronic delta hepatitis. In: The hepatitis delta virus and its infection, Rizzetto M, Gerin JL, Purcell RH (Eds), Alan R Liss, New York 1987. p.145
- Negro F, Rizzetto M. Diagnosis of hepatitis delta virus infection. J Hepatol 1995; 22:136
- Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018; 67:1560
- Abbas Z, Khan MA, Salih M, Jafri W. Interferon alpha for chronic hepatitis D. Cochrane Database Syst Rev 2011; :CD006002
- Ilan Y, Klein A, Taylor J, Tur-Kaspa R. Resistance of hepatitis delta virus replication to interferon-alpha treatment in transfected human cells. J Infect Dis 1992; 166:1164
- McNair AN, Cheng D, Monjardino J, et al. Hepatitis delta virus replication in vitro is not affected by interferon-alpha or -gamma despite intact cellular responses to interferon and dsRNA. J Gen Virol 1994; 75 ( Pt 6):1371
- Niro GA, Ciancio A, Gaeta GB, et al. Pegylated interferon alpha-2b as monotherapy or in combination with ribavirin in chronic hepatitis delta. Hepatology 2006; 44:713
- Rasshofer R, Choi SS, Wolfl P, et al. Inhibition of HDV RNA replication in vitro by ribavirin and suramin. In: Viral Hepatitis and Liver Disease, Hollinger FB, Lemon SM, Margolis HS (Eds), Williams & Wilkins, Baltimore 1991. p.659.
- Buti M, Lopez-Talavera JC, Allende H, et al. Serological diagnosis of chronic delta infection: correlation between serological markers and hepatitis delta virus RNA in hepatic tissue. Prog Clin Biol Res 1993; 382:319.
- Garripoli A, Di Marco V, Cozzolongo R, et al. Ribavirin treatment for chronic hepatitis D: a pilot study. Liver 1994; 14:154.
- Petcu DJ, Aldrich CE, Coates L, et al. Suramin inhibits in vitro infection by duck hepatitis B virus, Rous sarcoma virus, and hepatitis delta virus. Virology 1988; 167:385.
- Ponzetto A, Negro F, Gerin JL, Purcell RH. Experimental hepatitis delta virus infection in the animal model. Prog Clin Biol Res 1991; 364:147.
- Lau DT, Doo E, Park Y, et al. Lamivudine for chronic delta hepatitis. Hepatology 1999; 30:546.
- Niro GA, Ciancio A, Tillman HL, et al. Lamivudine therapy in chronic delta hepatitis: a multicentre randomized-controlled pilot study. Aliment Pharmacol Ther 2005; 22:227.
- Wolters LM, van Nunen AB, Honkoop P, et al. Lamivudine-high dose interferon combination therapy for chronic hepatitis B patients co-infected with the hepatitis D virus. J Viral Hepat 2000; 7:428.
- Yurdaydin C, Bozkaya H, Onder FO, et al. Treatment of chronic delta hepatitis with lamivudine vs lamivudine + interferon vs interferon. J Viral Hepat 2008; 15:314.
- Yurdaydin C, Bozkaya H, Gürel S, et al. Famciclovir treatment of chronic delta hepatitis. J Hepatol 2002; 37:266.
- Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem 1996; 65:241
- Koh C, Canini L, Dahari H, et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis 2015; 15:1167
- Ni Y, Lempp FA, Mehrle S, et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 2014; 146:1070.
- Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012; 1:e00049.
- Bazinet M, Pantea V, Placinta G, et al. Update on safety and efficacy in the REP 401 protocol: REP 2139-Mg or REP 2165-Mg used in combination with tenofovir disoproxil fumarate and pegylated interferon alpha-2a in treatment naive Caucasian patients with chronic HBeAg negative HBV infection. International Liver Congress; Amsterdam, Netherlands; April 19–23, 2017.
Bazinet M, Pântea V, Cebotarescu V, et al. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2017; 2:877.
