EPIDEMIOLOGY
Chronic hepatitis C virus (HCV) infection is one of the most common chronic liver disease and accounts for 8000 to 13,000 deaths each year. Globally, it was estimated that in 2005, more than 185 million people had hepatitis C virus (HCV) antibodies (prevalence of 2.8 percent).(1)
HCV is highly endemic in Pakistan, where a national survey, conducted in 2007–2008, estimated HCV prevalence at 4.8%.(2) In Pakistan 10 million people are presumed to be infected with HCV.(3) Genotype 3 predominates (79%) followed by genotype 1 (~10%).(4)
PATHOPHYSIOLOGY(5)
Hepatitis C virus (HCV) is a spherical, enveloped, single-stranded RNA virus. The natural targets of HCV are hepatocytes and, possibly, B lymphocytes. Viral clearance is associated with the development and persistence of strong virus-specific responses by cytotoxic T lymphocytes and helper T cells.
In most infected people, viremia persists and is accompanied by variable degrees of hepatic inflammation and fibrosis. Findings from studies suggest that at least 50% of hepatocytes may be infected with HCV in patients with chronic hepatitis C.
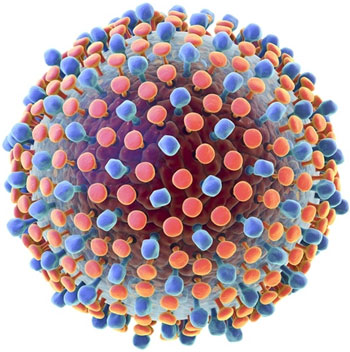
The proteolytic cleavage of the virus results in two structural envelope glycoproteins (E1 and E2) and a core protein.(6) Two regions of the E2 protein, designated hypervariable regions 1 and 2, have an extremely high rate of mutation, believed to result from selective pressure by virus-specific antibodies. The envelope protein E2 also contains the binding site for CD-81, a tetraspanin receptor expressed on hepatocytes and B lymphocytes that acts as a receptor or coreceptor for HCV.
Other viral components are nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A, NS5B, and p7), whose proteins function as helicase-, protease-, and RNA-dependent RNA polymerase, although the exact function of p7 is unknown. These nonstructural proteins are necessary for viral propagation and have been the targets for newer antiviral therapies, such as the direct-acting antiviral agents (DAAs). NS2/3 and NS3/4A are proteases responsible for cleaving the HCV polyprotein. NS5A is critical for the assembly of the cytoplasmic membrane-bound replication complex; one region within NS5A is linked to an interferon (IFN) response and is called the IFN sensitivity–determining region. NS5B is an RNA dependent RNA polymerase required for viral replication; it lacks proofreading capabilities and generates a large number of mutant viruses known as quasispecies. These represent minor molecular variations with only 1%-2% nucleotide heterogeneity.
Genotypes
HCV genomic analysis by means of an arduous gene sequencing of many viruses has led to the division of HCV into six genotypes based on homology. Numerous subtypes have also been identified. Arabic numerals denote the genotype, and lower-case letters denote the subtypes for lesser homology within each genotype.(7) Genotype details are as follows:
- Genotype 1a occurs in 50%-60% of patients in the United States.
- Genotype 1b occurs in 15%-20% of patients in the United States; this type is most prevalent in Europe, Turkey, and Japan.
- Genotype 1c occurs in less than 1% of patients in the United States.
- Genotypes 2a, 2b, and 2c occur in 10%-15% of patients in the United States; these subtypes are widely distributed and are most responsive to medication.
- Genotypes 3a and 3b occur in 4%-6% of patients in the United States; these subtypes are most prevalent in India, Pakistan, Thailand, Australia, and Scotland.
- Genotype 4 occurs in less than 5% of patients in the United States; it is most prevalent in the Middle East and Africa.
- Genotype 5 occurs in less than 5% of patients in the United States; it is most prevalent in South Africa.
- Genotype 6 occurs in less than 5% of patients in the United States; it is most prevalent in Southeast Asia, particularly Hong Kong and Macao.
TRANSMISSION
Injection drug use: Parenteral exposure to the hepatitis C virus is the most efficient means of transmission. Thus, it is not surprising that injection drug use with shared needles or other paraphernalia has been the most common identifiable source of acute HCV infection.
HCV infection also has been associated with a history of intranasal cocaine use, presumably due to blood on shared straws.(8)
Blood transfusion: Blood transfusion was a major risk factor for acute infection in the past, with more than 10 percent of transfusion recipients acquiring infection in some studies.(9) The screening of blood donors for historical risk factors, serologic evidence of hepatitis B infection (HBsAg and anti-HBc), and elevated serum ALT caused a striking reduction in the rates of non-A, non-B post-transfusion hepatitis, even before HCV was identified. The subsequent initiation of donor screening for anti-HCV antibodies in 1990 has nearly eliminated the risk of post-transfusion acute HCV infection. The estimated risk is now less than one in a million per unit transfused.(10)
Health care workers: Transmission of HCV to healthcare workers may occur via needle-stick injuries or other occupational exposures. Needle-stick injuries in the healthcare setting result in a 3% risk of HCV transmission.
Health care-associated: Nosocomial transmission of HCV has been documented in several health care settings.(11-13)
Organ transplantation: Transplant recipients who receive organs from HCV-positive donors have a high risk of acquiring HCV infection and liver disease.(14-16)
Sexual or household contact: The efficiency of HCV transmission by sexual or household contact is low. The majority of the data does not support transmission to nonsexual partners.(17-19) The seroprevalence of anti-HCV is increased among heterosexuals with many partners and men who have sex with men (MSM), groups which serve as an epidemiologic barometer of sexual transmission risk.(20-22) The risk of sexual transmission may be higher if the index case is coinfected with HIV.(20)
Patients with acute or chronic HCV infection should be advised that transmission to sexual or household contacts is a possibility, although the risk is relatively low. It is likely that the use of condoms will lower the risk of sexual transmission further, similar to the case with hepatitis B virus and HIV.
Perinatal transmission: Perinatal transmission of HCV occurs at the time of birth in about 5 percent of infants born to anti-HCV positive women.(23) The risk of infection is approximately twofold higher in infants born to women coinfected with HCV and HIV.(24) Transmission occurs almost exclusively from mothers who are HCV-RNA positive (as opposed to those who are anti-HCV positive but HCV-RNA negative).(25) Breastfeeding is not associated with transmission.
Hemodialysis: HCV infection is more common among patients on dialysis than in the general population. Although the incidence of HCV infection has been declining among dialysis patients, the relatively high incidence of anti-HCV seropositivity in this population remains a concern and intermittent outbreaks in dialysis units continue to occur.(26) A number of risk factors have been identified for HCV infection among dialysis patients, including blood transfusions, the duration of end-stage renal disease (and dialysis), the type of dialysis (risk is highest with in-hospital hemodialysis and lowest with peritoneal dialysis), and the prevalence of HCV infection in the dialysis unit.
Other: Procedures involved in traditional medicine, folk medicine (e.g. scarification, cupping), tattooing, body piercing, and commercial barbering may also transmit HCV on rare occasions. Tattooing and body piercing have the potential to transmit HCV. However, the extent to which they contribute to the disease burden of HCV is uncertain.
Surprisingly high rates of HCV infection (approximately 30 percent) have been found in patients with alcohol abuse, even in the absence of other risk factors for infection.(27-30)
NATURAL HISTORY
The majority of patients who acquire HCV do not spontaneously clear the virus and thus develop chronic HCV infection. Chronic infection results in liver fibrosis and ultimately cirrhosis in a subset of patients, although the rate of disease progression is variable. Patients who develop cirrhosis are at further risk for complicating events (such as variceal hemorrhage, ascites, and encephalopathy) and hepatocellular carcinoma, although many patients with compensated cirrhosis remain stable for years.
Risk of chronic infection: The risk of chronic infection after HCV acquisition is high. In most studies, 50 to 85 percent of patients chronically remain HCV RNA positive following infection and seroconversion, depending on the population and the source of infection.(31) The mechanism responsible for the high prevalence of viral persistence, and thus chronic infection, is unclear, but both viral and host factors are likely to contribute.
Acute exacerbation of chronic infection: Acute exacerbation of chronic HCV infection, with a significant elevation of serum aminotransferase levels over the baseline level in the absence of other potential causes of acute hepatitis, can occur. However, this phenomenon is not well characterized, and there are no standard definitions for it. Thus, its true incidence is unknown. Widely fluctuating aminotransferases were reported frequently in early studies on HCV, with an approximate incidence of ten percent.(32)
Risk and rate of progression to cirrhosis: The natural history of chronic HCV infection has been difficult to clearly define because of the long course of the disease, the difficulty in measuring precise duration of infection, and other factors that can affect disease course. A systematic review of 111 studies analyzing the natural history of HCV infection estimated that the prevalence of cirrhosis 20 years after infection was 16 percent (95% CI 14-19 percent).(33) Estimates of the rate of cirrhosis development, however, have varied widely, in part because of the different study populations that may have had variable risk factors for fibrosis progression.(34-50)
Studies of patients who presented clinically with chronic hepatitis tend to report a more aggressive course with a high risk of cirrhosis (and subsequent consequences of decompensation and hepatocellular carcinoma).(34,39,41)
Hepatic decompensation: Hepatic decompensation is characterized by the development of certain liver-related complications, including ascites, variceal bleeding, and encephalopathy. In patients with chronic HCV infection, jaundice is almost always a sign of advanced liver disease. Almost all HCV-infected patients who develop these complications have cirrhosis; however, not all patients with cirrhosis develop these complications.(51-53)
Hepatocellular carcinoma: HCV-associated mortality is more likely to be due to end stage liver disease rather than hepatocellular carcinoma. Estimates of the risk of developing hepatocellular carcinoma once cirrhosis has developed have varied from 0 to 3 percent per year in various reports.(51,52) The risk appears to be greater with genotype 1b compared with genotypes 2a/c, although this observation may be confounded by other factors.(54)
In contrast to hepatitis B virus infection, hepatocellular carcinoma in patients with HCV occurs almost exclusively in those with cirrhosis, suggesting that cirrhosis is the major risk factor.
SIGN AND SYMPTOMS(55)
Although many patients with chronic HCV infection are symptomatic, most symptoms are nonspecific and not clearly a result of HCV infection itself. Even if cirrhosis develops, many patients have only nonspecific symptoms. Occasionally, patients have specific extrahepatic findings (such as cryoglobulinemia, renal disease, or specific dermatologic disorders) that are directly related to HCV infection.
Generalized symptoms: Patients with chronic HCV infection often have a high symptom burden, but the extent to which HCV infection itself, rather than comorbid conditions, contributes to the symptoms is unclear. The most frequent complaints are fatigue and sleep disturbances; other symptoms include nausea, diarrhea, abdominal pain, anorexia, myalgia, arthralgia, weakness, and weight loss.(56) Neuropsychiatric symptoms (e.g. depression and anxiety) are also common.
Symptoms may lead to a decrease in the quality of life,(57) which may in part be accounted for by awareness of infection,(58) and which can be improved following successful treatment.(59)
HCV infection has also been associated with cognitive impairment, which has been demonstrated in patients with HCV independent of the severity of liver disease.(60-63)
Extrahepatic manifestations: A number of extrahepatic diseases have been associated with chronic HCV infection. Most cases appear to be directly related to the viral infection. These include:
- Hematologic diseases, such as essential mixed cryoglobulinemia and lymphoma
- Renal disease, particularly membranoproliferative glomerulonephritis
- Autoimmune disorders, such as thyroiditis and the presence of autoantibodies
- Dermatologic conditions, such as porphyria cutanea tarda and lichen planus
- Diabetes mellitus
Laboratory findings
Serum aminotransferases: There is wide variability in serum aminotransferase levels among individual patients with chronic HCV infection over time. Up to one-third of patients have a normal serum alanine aminotransferase (ALT).(64) Slight enzyme elevations are usually seen in the remaining patients; only about 25 percent have a serum ALT concentration more than twice normal, and it is rare to find elevations more than 10 times normal.
Occasionally, acute increases in the serum aminotransferases can occur during chronic HCV infection without apparent alternate cause. This phenomenon is not well defined, and so the incidence is difficult to assess.
Viral levels: During chronic HCV infection (i.e. following the acute phase), viral levels of HCV remain generally constant, although significant fluctuations can occur.(65-67) There is little correlation between HCV viral levels and serum aminotransferase levels or the severity of liver disease.(68-70) However, the viral level may influence the optimal duration of certain antiviral regimens.
The variables that affect viral levels are not well elucidated. Coinfections with other viruses have been observed to impact the serum levels of HCV RNA. HCV levels generally increase following HIV infection.(71,72) On the other hand, a persistent decline in HCV RNA replication can occur following acute hepatitis B virus (HBV) infection.(73) Nevertheless, coinfection with either HIV or HBV is associated with a faster rate of fibrosis progression.
Other: Other laboratory manifestations that can be observed in chronic HCV infection include those that are related to the potential extrahepatic manifestations of HCV. As examples, low platelets may reflect immune-mediated thrombocytopenia; a reactive rheumatoid factor, an increased production of autoantibodies; and proteinuria and/or microscopic hematuria, glomerulonephritis.
Findings in cirrhosis
Approximately 5 to 30 percent of chronically infected individuals develop cirrhosis over a 20- to 30-year period of time. The development of cirrhosis is silent in the majority of patients in whom it occurs.(34) Although these patients tend to be more symptomatic than those with chronic hepatitis alone, no clinical symptom, physical sign, or laboratory test is either sensitive or very specific for the diagnosis. The physical examination may reveal hepatomegaly (68 percent in one series) or splenomegaly.(34)
Laboratory testing can be helpful in identifying cirrhosis in HCV-infected patients, but none are 100 percent specific. Suggestive findings include an elevation in the serum bilirubin concentration, hypoalbuminemia, or a decrease in the platelet count.(74,75)
The serum alpha fetoprotein (AFP) concentration may be mildly elevated in chronic HCV infection and does not necessarily imply the presence of hepatocellular carcinoma or cirrhosis; up to 43 percent of patients with cirrhosis without hepatocellular carcinoma have a serum AFP between 10 and 100 ng/mL.(51,76) Nevertheless, an elevated serum AFP concentration requires imaging of the liver to rule out hepatocellular carcinoma. Serial testing for several months is warranted if the imaging studies are negative, since rising levels may be indicative of an occult malignancy.
- Clinical or biochemical evidence of chronic liver disease (eg, persistently elevated alanine aminotransferase)
- Extrahepatic manifestations of chronic HCV infection, including:
- Porphyria cutanea tarda
- Mixed cryoglobulinemia
- Lichen planus
- Necrolytic acral erythema
- Unexplained arthritis or false-positive rheumatoid factor
- Sjögren’s syndrome/sicca symptoms
- Membranoproliferative glomerulonephritis
- Idiopathic thrombocytopenic purpura
- Receipt of clotting factors made prior to the introduction of sensitive screening of the supply (1987 in the United States)
- Receipt of blood or organs prior to the introduction of sensitive screening of the supply (July 1992 in the United States)
- Receipt of blood from a donor later diagnosed with HCV
- Other risk for receipt of potentially contaminated blood products (eg, care in neonatal intensive care prior to sensitive screening)
- Born between 1945 and 1965 and living in the United States
- HIV infected
- Men who have sex with men (MSM)
- Past or present use of chronic hemodialysis (or upon initiation of maintenance hemodialysis)
- History of or present incarceration
- Residence in a high-prevalence country
- Birth to HCV-infected mother
- Current sexual partnership with an HCV-infected individual
- Needlestick injury or mucosal exposure to HCV-infected blood
- Percutaneous exposure in unregulated setting
- Planned organ donation
DIAGNOSTIC TESTS
Diagnostic tests for hepatitis C virus (HCV) can be divided into two broad categories:
- Serologic assays that detect antibodies to hepatitis C
- Molecular assays that detect or quantify HCV RNA
Other investigations such as genotype testing, serum fibrosis panels and liver biopsy may help to predict the response to treatment and prognosis.
Diagnosis and Testing Approach:
The diagnosis of chronic HCV infection is usually made in a patient with a reactive HCV antibody test and a positive molecular test that detects the presence of HCV RNA.
Testing algorithm: Initial diagnostic evaluation for chronic HCV typically begins with an antibody test. A reactive or indeterminate/equivocal antibody test should be followed by HCV RNA testing. For patients who have a greater likelihood of false-negative antibody testing (e.g. severely immunocompromised or hemodialysis patients or those suspected of having acute hepatitis C), HCV RNA testing should be sent at the same time as antibody testing.
If HCV RNA is detected, the diagnosis of HCV infection is confirmed. If HCV RNA is not detected, then a reactive antibody likely represents either a past HCV infection that subsequently was cleared or a false-positive antibody test. The different testing outcomes are discussed in further detail below.
Several different antibody tests are available, including laboratory based immunoassays, rapid point-of-care tests, and home-based tests, and all can be used as the initial assay for antibody testing for HCV.
Quantitative HCV RNA tests used to confirm the diagnosis should have a detection level of 25 international units/mL or lower. If the available quantitative test does not have that level of sensitivity, then a qualitative test should be used for diagnosis.
These recommendations are consistent with the joint HCV guidelines from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.(77)
In resource-limited locations or other settings where HCV RNA testing is not accessible, HCV core antigen testing may be a more affordable alternative, if available.
Nonreactive anti-HCV antibody: If the antibody test is nonreactive, then chronic HCV infection is unlikely and testing can stop.
In occasional situations, however, patients may lack detectable levels of anti-HCV antibodies despite having an HCV infection, and thus testing for HCV RNA despite a nonreactive antibody test is important to exclude infection. These include severely immunocompromised patients, patients on hemodialysis, and those who are suspected of having acute HCV infection because of symptoms or recent exposures.
Severely immunocompromised and hemodialysis patients: Patients on hemodialysis, transplant recipients, and those with advanced HIV infection may have a higher rate of false-negative antibody testing than immunocompetent patients.(78,79)
Thus, HCV RNA testing if often performed to evaluate for HCV infection even if the HCV antibody test is nonreactive in such patients, particularly if the patient has an elevated transaminase level or other concern for chronic hepatitis. However, for patients who are undergoing serial screening for HCV infection (e.g. patients on maintenance hemodialysis), repeatedly checking HCV RNA levels despite negative HCV antibody tests is likely not necessary in the absence of concern for recent exposure.(80)
HCV infection is diagnosed if the HCV RNA is positive, even if the HCV antibody test is nonreactive.
Patients with acute hepatitis or recent exposure: In patients suspected of having acute HCV infection, either because of symptoms or signs consistent with acute hepatitis or because of a recent exposure, HCV RNA should be checked at the same time as antibody testing. Following exposure, HCV RNA becomes detectable prior to reactive antibodies. Most patients develop detectable antibodies between two and six months after exposure. Antibody testing is positive in 50 percent of patients with acute HCV infection at the time of presentation, and in 90 percent at some time during the acute illness.(81) Testing for HCV RNA allows earlier diagnosis.
Reactive antibody and positive RNA test: A positive HCV RNA result is evidence of HCV infection. Usually, patients who have both reactive anti-HCV antibody and detectable HCV RNA have chronic infection. However, in some cases, patients acutely infected with HCV will also have a reactive antibody test and positive HCV RNA. In these cases, the distinction between acute and chronic hepatitis C is difficult and must take into account recent exposures, the presence of symptoms, prior HCV and aminotransferase testing results, and patterns of HCV RNA levels over time. This is discussed in detail elsewhere.
For patients diagnosed with HCV infection, linking to medical care for further evaluation is important. This includes assessment of the extent of liver disease through physical exam, laboratory testing, and consideration of liver biopsy. Additionally, HCV RNA quantification (if diagnosis was made with a qualitative RNA test) and HCV genotype testing are important for patients in whom treatment is being considered.
Reactive antibody and negative RNA test: The absence of detectable HCV RNA essentially confirms the absence of chronic HCV infection. False-negative tests for RNA are unusual when sensitive quantitative or qualitative tests with a low level of detection (e.g. <50 international units/mL) are used.
In this situation, the reactive anti-HCV antibody most likely represents prior infection that subsequently cleared spontaneously (or following successful therapy) or a false-positive antibody test due to technical reasons. The estimated rate of spontaneous clearance of virus after infection is 20 to 45 percent depending upon the age and immune status of the individual at the time of infection.(81)
Other less frequent situations may result in a reactive antibody and negative RNA test:
- Detection of anti-HCV antibodies that have been passively acquired from blood transfusions. In this situation, anti-HCV disappears over the next few weeks in keeping with the half-life of IgG. This is now extremely unusual because of improved testing of the blood supply.
- Detection of maternal anti-HCV antibodies in babies.
- Recurrent episodes of viremia with genetic identity to the original infecting HCV strain have been described in injecting drug users who were thought to have cleared HCV.(82) It is unclear how frequent this phenomenon occurs.
- The amount of HCV RNA may be below the limit of detection of the assay or there may be other technical problems with the test. This is less of an issue when currently available sensitive qualitative (TMA) and quantitative (real-time PCR) assays are used.
Diagnostic Tests
Antibody testing
Antibodies to HCV can be detected using a number of assays, including standard immunoassays that are performed in a laboratory, rapid immunoassays that can be performed at the point-of-care, and home tests on specimens self-collected by the patient.
Standard immunoassay testing: The standard test used by most clinical laboratories to detect anti-HCV antibodies in serum and plasma is an immunoassay, which can be linked to various methods of signaling a positive test, including an enzymatic reaction (EIA, also called enzyme-linked immunosorbent assay or ELISA) or light emission (chemiluminescence immunoassay). These immunoassays have many advantages in the diagnostic setting, including ease of use, low variability, ease of automation, and relatively low expense.
Rapid immunoassay tests: Several rapid tests for HCV antibodies have been developed that have performance comparable to standard laboratory-based immunoassays. These tests can be run on venous blood, finger stick blood, serum, plasma, and oral fluid, and results are generally available in less than 30 minutes. The tests are designed for point-of-care testing to provide increased opportunities for HCV testing outside of traditional clinical settings.(83)
Self-collected tests: An over-the-counter antibody testing kit has been approved by the FDA. A sample is sent to the laboratory and results are returned within 4 to 10 business days. Data presented to the FDA suggest that the accuracy of the test is comparable to hospital laboratory-based antibody testing.
Recombinant immunoblot assay: The recombinant immunoblot assay (RIBA) is a test that detects HCV antibodies with similar sensitivity but higher specificity than screening second generation immunoassays. It is no longer available in many countries.
HCV RNA assays:
HCV RNA detection and quantification are essential tools in the diagnosis and management of individuals with chronic HCV infection. HCV RNA assays are used to confirm the presence or absence of infection and to quantify the amount of HCV RNA present, and may be used to guide decisions regarding duration of treatment with certain regimens.
Nucleic acid tests (NAT) for detection of HCV RNA have been traditionally divided into two categories: qualitative and quantitative assays. The lower limit of detection (i.e. the lowest viral level that can be detected) with most currently available quantitative tests is comparable to that of qualitative tests. Thus, quantitative tests can be used for detection of infection. Most patients with chronic HCV infection will have HCV RNA much greater than the lower level of detection of quantitative tests.
Methods of RNA detection: Several methods can be used to detect and measure HCV RNA and have varying levels of sensitivity. These include polymerase chain reaction (PCR) based methods, transcription mediated amplification (TMA), and branched DNA testing.
Quantitative tests: Quantitative assays assess the quantity of HCV RNA in international units/mL and vary in their limits of detection and dynamic range. Quantitative assays are used before treatment to measure baseline HCV viral load. Quantitative assays may also be used during and after treatment to assess on-treatment response and to assess response to therapy. However, most direct-acting antiviral (DAA) regimens with newly available DAAs have very high rates of sustained virologic response (SVR), and on-treatment monitoring of HCV viral load is not necessary.
Qualitative tests: Qualitative tests are capable of detecting low levels of HCV RNA and are used for confirming the diagnosis of HCV infection and assessing SVR to antiviral therapy. They provide results as positive or negative and some have a lower limit of detection as low as <10 international units/mL HCV RNA. Examples of qualitative HCV RNA assays are Amplicor PCR assay (Roche Diagnostics) and Versant TMA assay (Siemens Healthcare Diagnostics).
HCV core antigen test: Several immunoassays have been developed to detect the HCV core (HCV cAg) protein, a component of the viral particle.(84,85) In resource-limited settings where nuclear acid testing (NAT) is not available, WHO guidelines recommend that an HCV cAg test be used instead to confirm viremia.(86)
Additional Evaluation:
The most important aspects of initial care of the patient newly diagnosed with HCV involve evaluating the extent of liver damage and determining candidacy for treatment. Assessment for treatment candidacy involves evaluation for factors that predict response to therapy, comorbidities that increase the urgency of treatment, and comorbidities that would be contraindications or complications for therapy.
History and physical exam: Individuals with chronic hepatitis C should undergo a complete history and physical exam with a focus on assessing the extent of underlying liver disease and evaluating candidacy for treatment.
Thus, history should include questions regarding factors associated with accelerated disease progression, including alcohol use, metabolic complications associated with fatty liver, and menopausal status (in women), complications that would suggest underlying cirrhosis (e.g. ascites, hematemesis, and mental status changes), and factors that may affect candidacy for antiviral therapy, including underlying cardiopulmonary disease, past or present psychiatric problems, autoimmune diseases, and other comorbid conditions.
Physical examination should include evaluation for stigmata of advanced liver disease such as spider angiomata, palmar erythema, splenomegaly, jaundice, or caput medusa. However, clinicians should be aware that absence of any of these findings does not rule out the possibility of underlying cirrhosis. Signs of extrahepatic manifestations of HCV infection, such as porphyria cutanea tarda should also be sought.
Basic laboratory testing: Similarly, to assess the extent of underlying liver disease, evaluate for extrahepatic complications, and evaluate candidacy for treatment, initial laboratory testing should include:
- Serum aminotransferase activity
- Measures of synthetic function: Bilirubin, prothrombin time, and albumin
- Complete blood count
- Renal function and glucose
- Urinalysis
- 25-hydroxy vitamin D
- Pregnancy test for women of childbearing potential
Additionally, it is reasonable to evaluate for and exclude other causes of chronic liver disease, such as iron overload syndromes or autoimmune hepatitis, in patients with elevated aminotransferases.
Because of the association between HCV and certain types of renal disease (e.g. mixed cryoglobulinemia and membranoproliferative glomerulonephritis), HCV-infected patients should be screened for proteinuria, hematuria, hypertension, and renal function. Additional evaluation for cryoglobulinemia, complement levels, and rheumatoid factors, and potentially kidney biopsy may be warranted in the setting of significant proteinuria and/or impaired renal function.
HCV genotype testing:
Determination of HCV genotype, on which the regimen, dosing, and duration of therapy as well as likelihood of response depend, is essential to making decisions about treatment.
Assessment of fibrosis stage:
Knowledge of the stage of fibrosis provides important prognostic information and guides certain decisions regarding treatment. Fibrosis stage can be assessed indirectly through history, physical examination, laboratory tests, and other noninvasive studies (such as the FibroSure and ultrasound-based transient elastography) or, rarely, with liver biopsy.
Testing for HIV, hepatitis B, and hepatitis A:
Patients diagnosed with HCV should also be tested for HIV and hepatitis B due to the common modes of transmission. Patients who are not immune to hepatitis B should be vaccinated to protect the liver from additional insults. In addition, patients should be tested for hepatitis A and vaccinated if not immune.
PATIENT SELECTION FOR TREATMENT
All patients with virologic evidence of chronic HCV infection (i.e. detectable HCV viral level over a six-month period) should be considered for treatment. Hepatitis C guidelines propose that because all patients cannot receive treatment immediately upon the approval of new agents, priority should be given to those with the most urgent need. The recommendations include the following:(77)
- Patients with advanced fibrosis, those with compensated cirrhosis, liver transplant recipients, and those with severe extraheptic complications are to be given the highest priority for treatment.
- Based on available resources, patients at high risk for liver-related complications and severe extrahepatic hepatitis C complications should be given high priority for treatment.
- Treatment decisions should balance the anticipated reduction in transmission versus the likelihood of reinfection in patients whose risk of HCV transmission is high and in whom HCV treatment may result in a reduction in transmission (e.g. men who have high-risk sex with men, active injection drug users, incarcerated persons, and those on hemodialysis).
PATIENT SELECTION FOR TREATMENT
All patients with virologic evidence of chronic HCV infection (i.e. detectable HCV viral level over a six-month period) should be considered for treatment. Hepatitis C guidelines propose that because all patients cannot receive treatment immediately upon the approval of new agents, priority should be given to those with the most urgent need. The recommendations include the following:(77)
- Patients with advanced fibrosis, those with compensated cirrhosis, liver transplant recipients, and those with severe extraheptic complications are to be given the highest priority for treatment.
- Based on available resources, patients at high risk for liver-related complications and severe extrahepatic hepatitis C complications should be given high priority for treatment.
- Treatment decisions should balance the anticipated reduction in transmission versus the likelihood of reinfection in patients whose risk of HCV transmission is high and in whom HCV treatment may result in a reduction in transmission (e.g. men who have high-risk sex with men, active injection drug users, incarcerated persons, and those on hemodialysis).
THERAPY CONSIDERATION
Spontaneous resolution of acute HCV infection may occur in 15% to 50% of patients.(88) Monitoring for spontaneous clearance for a minimum of 6 months before initiating treatment is therefore recommended. Initiating treatment earlier for patients with lower stage fibrosis may extend the benefits of sustained virologic response (SVR).
Antiviral therapy for chronic hepatitis C should be determined on a case-by-case basis. However, treatment is widely recommended for patients with elevated serum alanine aminotransferase (ALT) levels who meet the following criteria:( 87)
- Age greater than 18 years
- Positive HCV antibody and serum HCV RNA test results
- Compensated liver disease (e.g. no hepatic encephalopathy or ascites)
- Acceptable hematologic and biochemical indices (hemoglobin at least 13 g/dL for men and 12 g/dL for women; neutrophil count >1500/mm3, serum creatinine < 1.5 mg/dL)
- Willingness to be treated and to adhere to treatment requirements
- No contraindications for treatment
TREATMENT OPTIONS
The following recommendations are based on guidelines from the American Association for the Study of Liver Diseases [AASLD], the Infectious Disease Society of America [ISDA], and the International Antiviral Society-USA [IAS-USA].(89)
- Treatment-naïve patients
- Genotype 1
- HCV genotype 1a treatment-naïve patients without cirrhosis
Recommended regimens
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) for 12 weeks if no baseline NS5A resistance-associated substitutions (RASs) for elbasvir are detected
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 8 weeks
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 8 weeks for non-black patients, non-HIV-infected persons, and those whose HCV RNA level is below 6 million IU/mL
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
Alternative regimens
- Daily fixed-dose combination of paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) with dasabuvir (600 mg) for 12 weeks as part of an extended-release regimen or plus twice-daily dosed dasabuvir (250 mg), with weight-based ribavirin
- Daily simeprevir (150 mg) plus sofosbuvir (400 mg) for 12 weeks
- Daily daclatasvir (60 mg) plus sofosbuvir (400 mg) for 12 weeks
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) with weight-based ribavirin for 16 weeks in patients who have baseline NS5A RASs for elbasvir
- HCV genotype 1a treatment-naïve patients with compensated cirrhosis
Recommended regimens
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) for 12 weeks in those with no baseline NS5A RASs for elbasvir
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
Alternative regimen
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) with weight-based ribavirin for 16 weeks in patients with baseline NS5A RASs for elbasvir
- HCV genotype 1b treatment-naïve patients without cirrhosis
Recommended regimens
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) for 12 weeks
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 8 weeks
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 8 weeks for non-black persons, non-HIV-infected patients, and those whose HCV RNA level is below 6 million IU/mL
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
Alternative regimens
- Daily fixed-dose combination of paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) for 12 weeks as part of an extended-release regimen or plus twice-daily dosed dasabuvir (250 mg)
- Daily simeprevir (150 mg) plus sofosbuvir (400 mg) for 12 weeks
- Daily daclatasvir (60 mg) plus sofosbuvir (400 mg) for 12 weeks
- HCV genotype 1b treatment-naïve patients with compensated cirrhosis
Recommended regimens
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) for 12 weeks
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
Alternative regimen
- Daily fixed-dose combination of paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) with dasabuvir (600 mg) for 12 weeks as part of an extended-release regimen or plus twice-daily dosed dasabuvir (250 mg)
- Genotype 2
- HCV genotype 2 treatment-naïve patients without cirrhosis
Recommended regimens
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 8 weeks
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
Alternative regimen
- Daily daclatasvir (60 mg) plus sofosbuvir (400 mg) for 12 weeks
- HCV genotype 2 treatment-naïve patients with compensated cirrhosis
Recommended regimens
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks
Alternative regimen
- Daily daclatasvir (60 mg) plus sofosbuvir (400 mg) for 16 to 24 weeks
- Genotype 3
- HCV genotype 3 treatment-naïve patients without cirrhosis
Recommended regimens
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 8 weeks
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
Alternative regimen
- Daily daclatasvir (60 mg) plus sofosbuvir (400 mg) for 12 weeks
- HCV genotype 3 treatment-naïve patients with compensated cirrhosis:
Recommended regimens
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
Alternative regimens
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) when Y93H is present
- Daily daclatasvir (60 mg) plus sofosbuvir (400 mg) for 24 weeks with or without weight-based ribavirin
- Genotype 4
- HCV genotype 4 treatment-naïve patients without cirrhosis
Recommended regimens
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 8 weeks
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) for 12 weeks
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks
Alternative regimen
- Daily fixed-dose combination of paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) and weight-based ribavirin for 12 weeks
- HCV genotype 4 treatment-naïve patients with compensated cirrhosis
Recommended regimens
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) for 12 weeks
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks
Alternative regimen
- Daily fixed-dose combination of paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) and weight-based ribavirin for 12 weeks
- Genotype 5 or 6
- HCV genotype 5 or 6 treatment-naïve patients with and without compensated cirrhosis
Recommended regimens
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 8 weeks in those without cirrhosis or for 12 weeks in those with cirrhosis
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks, regardless of cirrhosis status
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks, regardless of cirrhosis status.
- Treatment-experienced patients with previous treatment failure
- Genotype 1a
- HCV genotype 1a peginterferon (PEG-IFN)/ribavirin treatment-experienced patients without cirrhosis
Recommended regimens
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) for 12 weeks if no baseline NS5A RASs for elbasvir are detected
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 8 weeks
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
Alternative regimens
- Daily fixed-dose combination of paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) with dasabuvir (600 mg) as part of an extended-release regimen or plus twice-daily dasabuvir (250 mg, and weight-based ribavirin for 12 weeks
- Daily simeprevir (150 mg) plus sofosbuvir (400 mg) for 12 weeks
- Daily daclatasvir (60 mg) plus sofosbuvir (400 mg) for 12 weeks
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) with weight-based ribavirin for 16 weeks for patients with baseline NS5A RASs for elbasvir
- HCV genotype 1a PEG-IFN/ribavirin treatment-experienced patients with compensated cirrhosis
Recommended regimens
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) for 12 weeks if no baseline NS5A RASs for elbasvir are detected
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks
Alternative regimens
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) plus weight-based ribavirin for 12 weeks
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) with weight-based ribavirin for 16 weeks in patients who have baseline NS5A RASs for elbasvir
- Genotype 1b
- HCV genotype 1b PEG-IFN/ribavirin treatment-experienced patients without cirrhosis
Recommended regimens
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) for 12 weeks
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 8 weeks
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
Alternative regimens
- Daily fixed-dose combination of paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) with dasabubir (600 mg) for 12 weeks as part of an extended-release regimen or plus twice-daily dosed dasabuvir (250 mg) for 12 weeks
- Daily simeprevir (150 mg) plus sofosbuvir (400 mg) for 12 weeks
- Daily daclatasvir (60 mg) plus sofosbuvir (400 mg) for 12 weeks
- HCV genotype 1b PEG-IFN/ribavirin treatment-experienced with compensated cirrhosis
Recommended regimens
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) for 12 weeks
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks
Alternative regimens
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) plus weight-based ribavirin for 12 weeks
- Daily fixed-dose combination of paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) with dasabubir (600 mg) for 12 weeks as part of an extended-release regimen or plus twice-daily dosed dasabuvir (250 mg)
- HCV genotype 1 nonstructural protein 3 (NS3) protease inhibitor (telaprevir, boceprevir, or simeprevir) plus PEG-IFN/ribavirin treatment-experienced patients without cirrhosis
Recommended regimens
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks
Alternative regimens
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) with weight-based ribavirin for 12 weeks for all genotype 1b patients, and genotype 1a patients without baseline NS5A RASs for elbasvir. Extend this treatment to 16 weeks for genotype 1a patients who have baseline NS5A RASs for elbasvir.
- HCV genotype 1 NS3 protease inhibitor (telaprevir, boceprevir, or simeprevir) plus PEG-IFN/ribavirin treatment-experienced patients with compensated cirrhosis
Recommended regimens
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks
Alternative regimens
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) plus weight-based ribavirin for 12 weeks
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) with weight-based ribavirin for 12 weeks for all genotype 1b patients, and genotype 1a patients without baseline NS5A RASs for elbasvir. Extend this treatment to 16 weeks for genotype 1a patients who have baseline NS5A RASs for elbasvir.
- HCV genotype 1 non-NS5A inhibitor, sofosbuvir-containing treatment-experienced patients without cirrhosis
Recommended regimens
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) for 12 weeks for genotype 1a patients
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks, regardless of HCV subtype
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks for genotype 1b patients
Alternative regimen
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) plus weight-based ribavirin, except in simeprevir failures, for 12 weeks
- HCV genotype 1 non-NS5A inhibitor, sofosbuvir-containing treatment-experienced patients with compensated cirrhosis
Recommended regimens
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) for 12 weeks for genotype 1a patients
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks, regardless of HCV subtype
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks for genotype 1b patients
- HCV genotype 1 NS5A inhibitor, direct-acting antiviral agent (DAA) treatment-experienced patients with or without compensated cirrhosis
Recommended regimen
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) for 12 weeks
Alternative regimen
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg), except NS3/4 protease inhibitor inclusive DAA combination regimens, for 16 weeks
- Genotype 2
- HCV genotype 2 PEG-IFN/ribavirin treatment-experienced patients without cirrhosis
Recommended regimens
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 8 weeks
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
Alternative regimen
- Daily daclatasvir (60 mg) plus sofosbuvir (400 mg) for 12 weeks
- HCV genotype 2 PEG-IFN/ribavirin treatment-experienced patients with compensated cirrhosis
Recommended regimens
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks
Alternative regimen
- Daily daclatasvir (60 mg) plus sofosbuvir (400 mg) for 16 to 24 weeks
- HCV genotype 2 sofosbuvir plus ribavirin treatment-experienced patients with or without compensated cirrhosis
Recommended regimens
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks
- Genotype 3
- HCV genotype 3 PEG-IFN/ribavirin treatment-experienced patients without cirrhosis
Recommended regimen
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
Alternative regimens
- Daily daclatasvir (60 mg) plus sofosbuvir (400 mg) for 12 weeks
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 16 weeks
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) for 12 weeks when RAS testing shows Y93H
- HCV genotype 3 PEG-IFN/ribavirin treatment-experienced patients with compensated cirrhosis
Recommended regimens
- Daily fixed-dose elbasvir (50 mg)/grazoprevir (100 mg) plus sofosbuvir (400 mg) for 12 weeks
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) for 12 weeks
Alternative regimens
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) with weight-based ribavirin for 12 weeks
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 16 weeks
- HCV genotype 3 DAA treatment (including NS5A inhibitors)-experienced patients with or without compensated cirrhosis
Recommended regimens
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) for 12 weeks
- Weight-based ribavirin for 12 weeks is recommended for individuals with prior NS5A inhibitor failure and cirrhosis
- Genotype 4
- HCV genotype 4 PEG-IFN/ribavirin treatment-experienced patients without cirrhosis
Recommended regimens
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 8 weeks
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) for 12 weeks for patients who had virologic relapse after previous PEG-IFN/ribavirin treatment
- Daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks
Alternative regimens
- Daily fixed-dose combination of paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) and weight-based ribavirin for 12 weeks
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) plus weight-based ribavirin for 16 weeks patients with previous on-treatment virologic failure (failure to suppress or breakthrough) while on PEG-IFN/ribavirin
- HCV genotype 4 PEG-IFN/ribavirin treatment-experienced patients with compensated cirrhosis
Recommended regimens
- Daily fixed-dose combination of sofosbuvir (400 mg)velpatasvir (100 mg) for 12 weeks
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) for 12 weeks for individuals who had virologic relapse after previous PEG-IFN/ribavirin treatment
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks
Alternative regimens
- Daily fixed-dose combination of paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) and weight-based ribavirin for 12 weeks
- Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) plus weight-based ribavirin for 16 weeks for patients with previous on-treatment virologic failure (failure to suppress or breakthrough) while on PEG-IFN/ribavirin
- Daily ledipasvir (90 mg)/sofosbuvir (400 mg) plus weight-based ribavirin for 12 weeks
- HCV genotype 4 DAA treatment (including NS5A inhibitors)-experienced patients with or without compensated cirrhosis
Recommended regimen
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) for 12 weeks
- Genotype 5 and 6
- HCV genotype 5 or 6 PEG-IFN/ribavirin treatment-experienced patients with or without compensated cirrhosis
Recommend regimens
- Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 8 weeks for patients without cirrhosis or for 12 weeks for patients with compensated cirrhosis
- Daily fixed-dose combination ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks
- HCV genotype 5 or 6 DAA treatment (including NS5A inhibitors)-experienced patients with or without compensated cirrhosis
Recommended regimen
- Daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) for 12 weeks
Treatment Classes of Hepatitis C
NS3/4A Protease Inhibitors:
NS3/4A protease inhibitors are inhibitors of the NS3/4A serine protease, an enzyme involved in post-translational processing and replication of HCV. Protease inhibitors disrupt HCV by blocking the NS3 catalytic site or the NS3/NS4A interaction.(90)
From following protease inhibitors only voxilaprevir is available in Pakistan.
Glecaprevir: Glecaprevir is a potent, pangenotypic protease inhibitor, which is only available in combination with the NS5A inhibitor pibrentasvir.
Grazoprevir: Grazoprevir is a potent, pangenotypic protease inhibitor, which is only available in combination with the NS5A inhibitor elbasvir.
Paritaprevir: Paritaprevir is an HCV protease inhibitor that is given with low dose ritonavir (a protease inhibitor that does not have anti-HCV activity) for a pharmacologic boosting effect. Paritaprevir and ritonavir are available as a fixed-dose combination with ombitasvir (an NS5A inhibitor) and usually given with the non-nucleoside NS5B inhibitor dasabuvir.
Simeprevir: Simeprevir was the first available second-generation protease inhibitor.(91) It is used in combination with peginterferon and ribavirin or in combination with sofosbuvir with or without ribavirin for chronic genotype 1 infection.
Voxilaprevir: Voxilaprevir is a potent, pangenotypic protease inhibitor, which is only available in combination with the NS5B inhibitor sofosbuvir and the NS5A inhibitor velpatasvir.
NS5A Inhibitors:
The NS5A protein plays a role in both viral replication and the assembly of the hepatitis C virus (HCV). However, the precise molecular mechanisms by which NS5A accomplishes these functions are uncertain. Thus, the exact mechanism of action of HCV NS5A inhibitors is unclear.
Daclatasvir: Daclatasvir is a NS5A inhibitor that is used mainly in combination with sofosbuvir.
Elbasvir: The NS5A inhibitor elbasvir is only available as a fixed-dose combination with the protease inhibitor grazoprevir.
Ledipasvir: Ledipasvir is the first NS5A inhibitor.It is available as part of a fixed-dose combination with sofosbuvir.
Ombitasvir: The NS5A inhibitor ombitasvir is only available as a fixed-dose combination with the protease inhibitors paritaprevir and ritonavir, which is usually given in combination with the non-nucleotide NS5B inhibitor dasabuvir.
Pibrentasvir: The pangenotypic NS5A inhibitor pibrentasvir is only available as a fixed-dose combination with the protease inhibitor glecaprevir.
Velpatasvir: The pangenotypic NS5A inhibitor velpatasvir is only available as a fixed-dose combination with the NS5B inhibitor sofosbuvir.
NS5B RNA-Dependent RNA polymerase inhibitors
NS5B is an RNA-dependent RNA polymerase involved in post-translational processing that is necessary for replication of HCV. There are two classes of polymerase inhibitors: nucleoside/ nucleotide analogues (NPIs) and non-nucleoside analogues (NNPIs). The NPIs target the catalytic site of NS5B and result in chain termination, while NNPIs act as allosteric inhibitors.
Nucleot(s)ide polymerase inhibitors (NPIs): Nucleotide inhibitors are activated within the hepatocyte through phosphorylation to nucleoside triphosphate, which competes with nucleotides, resulting in chain termination during RNA replication of the viral genome.
Sofosbuvir: It is indicated for treatment of CHC infection genotypes 1, 2, 3, and 4, 5, 6 as part of a combination antiviral regimen, including those with hepatocellular carcinoma meeting Milan criteria (awaiting liver transplantation) to prevent HCV recurrence and those with HCV/HIV-1 co-infection.
Non-nucleoside polymerase inhibitors (NNPIs): As a class, NNPIs are less potent, are more genotype specific, have a low to moderate barrier to resistance, and have variable toxicity profiles.
Dasabuvir: Dasabuvir is administered and packaged with ombitasvir-paritaprevir-ritonavir.
Fixed-Dose Combinations
Elbasvir-grazoprevir:
Elbasvir, an NS5A inhibitor, and grazoprevir, a NS3/4A protease inhibitor with a high barrier of resistance, are coformulated in a single tablet. This regimen is administered with or without weight-based ribavirin, depending on certain patient characteristics. It is indicated for chronic hepatitis C virus genotype 1 infection and genotypes 4, 5, and 6 infection in adults.
Glecaprevir-pibrentasvir:
The NS3/4A protease inhibitor glecaprevir and the NS5A inhibitor pibrentasvir are coformulated in a single tablet. The regimen is pangenotypic and has a high barrier to resistance.
Ledipasvir-sofosbuvir:
The NS5A inhibitor ledipasvir and the nucleotide polymerase (NS5B) inhibitor sofosbuvir are coformulated in a single tablet. This regimen is administered with or without weight-based ribavirin, depending on the patient population. It is indicated for chronic hepatitis C virus genotype 1 infection and genotypes 4, 5, and 6 infection in adults.
Ombitasvir-paritaprevir-ritonavir with or without dasabuvir:
The NS5A inhibitor ombitasvir, the HCV protease inhibitor paritaprevir, and the protease inhibitor ritonavir are coformulated in a single tablet. Ritonavir does not have direct anti-HCV activity but instead is included to increase levels of paritaprevir through inhibition of CYP3A-mediated metabolism. It is indicated in chronic hepatitis C virus genotype 1 infection and genotypes 4, infection in adults.
Sofosbuvir-velpatasvir:
The pangenotypic NS5A inhibitor velpatasvir and the NS5B inhibitor sofosbuvir are coformulated in a single tablet.
Sofosbuvir-velpatasvir-voxilaprevir:
This combination adds the NS3/4A inhibitor voxilaprevir to the NS5B inhibitor sofosbuvir and the NS5A inhibitor velpatasvir. This regimen is pangenotypic and mainly reserved for patients who have prior failure on DAA (and predominantly NS5A inhibitor-containing) regimens.
Other treatment options:
Interferons: It is no more recommended. They are naturally produced proteins with antiviral, antitumoral, and immunomodulatory actions. Interferons alfa, beta, and gamma may be given topically, systemically, and intralesionally. Interferons are immunomodulators that may shorten the clinical course, prevent complications, prevent latent and/or subsequent recurrences, decrease transmission, and eliminate established latency.
PEG-IFN alfa-2a- consists of IFN alfa-2a attached to a 40-kd branched PEG molecule. It is predominantly metabolized by the liver. The adult dosage is 180 mcg/kg SC once weekly.
PEG-IFN alfa-2b- consists of IFN alfa-2b attached to a single 12-kd PEG chain. It is excreted by the kidneys. PEG-IFN has sustained absorption, a slower rate of clearance, and a longer half-life than unmodified IFN, which permits more convenient once-weekly dosing and significantly improves quality of life for patients. The adult dose is 1.5 mcg/kg SC.
Ribavirin: is an antiviral nucleoside analogue. Its chemical name is D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide. Given alone, ribavirin has little effect on the course of hepatitis C. Given in combination with interferon and DAA, it significantly augments the rate of sustained virologic response. The adult dosage is 10.6 mg / kg orally once daily or in 2 divided doses.
GOALS OF HCV TREATMENT
The goal of antiviral therapy in patients with chronic hepatitis C virus (HCV) is to eradicate HCV RNA, which is predicted by attainment of a sustained virologic response (SVR), defined as an undetectable RNA level 12 weeks following the completion of therapy.
An SVR is associated with a 97 to 100 percent chance of being HCV RNA negative during long-term follow-up and can therefore be considered cure of the HCV infection. Attaining an SVR (with direct-acting antiviral [DAA] regimens as well as with interferon-based regimens) has been associated with decreases in all-cause mortality, liver-related death, need for liver transplantation, hepatocellular carcinoma rates, and liver-related complications, even among those patients with advanced liver fibrosis.
GUIDELINES
Treatment for chronic HCV is based on guidelines from the prestigious societies such as EASL, AASLD, WHO.
American Association for the Study of Liver Diseases is the leading organization of scientists and health care professionals committed to preventing and curing liver disease.
To review the AASLD guidelines, click on the link below.
https://www.hcvguidelines.org/
European Association for the Study of the Liver is a major European Association with international influence dedicated to the liver and liver disease.
To review the EASL guidelines, clink on the link below.
http://www.easl.eu/research/our-contributions/clinical-practice-guidelines/detail/easl-recommendations-on-treatment-of-hepatitis-c-2018
WHO Guidelines for the screening, Care and treatment of persons with Hepatitis C infection,
http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/
TREATMENT RESPONSE
Virologic Breakthrough: A virologic breakthrough refers to the reappearance of HCV RNA while still on therapy in a patient who had suppressed their viral level earlier in the course of therapy
Nonresponse: The term nonresponse refers to patients who do not achieve an undetectable HCV RNA during the first 24 weeks of treatment. There are two forms of virologic non-response that are important to distinguish from each other: null response and partial response.
Null Response: The term null response is a subcategory of nonresponse and refers to the situation when a patient does not suppress their HCV level by at least 2 log10 by week 12 of treatment.
Partial Response: The term partial response is a subcategory of nonresponse and describes a decrease in HCV RNA levels by at least 2 log10 at week 12 of treatment, but detectable levels at week 24.
End-of-Treatment Response (ETR): An end-of-treatment response (ETR) refers to an undetectable HCV RNA level at the end of the course of therapy.
Sustained virologic response at Week 24 (SVR24): Sustained virologic response at week 24 is defined as an undetectable HCV RNA level 24 weeks after treatment discontinuation.
Sustained virologic response at Week 12 (SVR12): Sustained virologic response at week 12 is defined as an undetectable HCV RNA level 12 weeks after treatment discontinuation.
Virologic Relapse: A virologic relapse occurs when HCV RNA reappears in a patient who had an end-of-treatment response.
Other Terms
With the advent of DAAs, response guided therapy is no more required but below are some of the response one should be aware while treating HCV Patients.
Rapid Virologic Response (RVR): A rapid virologic response, or RVR, is defined as an undetectable HCV RNA at week 4.
Extended Rapid Virologic Response (eRVR): Patients who have an undetectable HCV RNA level at week 4 through week 12 are considered to have an extended rapid virologic response (eRVR).
Very Rapid Virologic Response (vRVR): In some research studies, investigators have now started to measure HCV RNA responses 2 weeks after initiating therapy and patients who have undetectable HCV RNA levels at week 2 are considered to have a very rapid virologic response (vRVR)
CONSULTATION AND LONG TERM MONITORING
Follow-up after antiviral therapy:
Virologic response to treatment should be assessed by checking the viral load at 12 weeks following the cessation of therapy. Sustained virologic response (SVR) is defined by an undetectable viral level at this time point, which is generally maintained through 24 weeks following the cessation of therapy and beyond. However, a very small proportion of patients (less than 1 percent in studies of direct-acting antivirals) experience virologic relapse between weeks 12 and 24, and some of those cases may be reinfection rather than true relapse. Thus, some practitioners also check the viral load at 24 weeks to ensure maintenance of SVR. Although SVR reflects effective cure of hepatitis C virus (HCV) infection, it does not confer immunity to HCV, and patients should be counseled that they are at risk for reinfection with future exposure.
Patients who achieve an SVR and do not have bridging fibrosis or cirrhosis do not require any specific follow-up for their HCV infection, though some will check an HCV viral load one year after the completion of treatment to confirm that the viral load remains undetectable.
Patients who fail to achieve an SVR should continue to be followed for signs of progression of liver disease and assessed for retreatment of HCV infection.
Patients with advanced fibrosis or cirrhosis, regardless of whether they attain an SVR, require ongoing monitoring (including liver ultrasonography every six months) because they continue to be at risk of hepatocellular carcinoma and other complications
Consultation
Psychosocial issues: Although most patients with chronic hepatitis C virus (HCV) infection are asymptomatic at the time of diagnosis, the potential sequelae of chronic HCV infection are significant, and this possibility can have important emotional and physical consequences. Counseling and screening for depression should be a major consideration, both at diagnosis and during subsequent follow-up. Many patients benefit from participation in a support group.
HCV-infected individuals may also have issues with ongoing illicit drug use. Such patients should be counseled on substance use treatment, including psychiatric services or opioid substitution therapy. Active injection drug use is not a contraindication to antiviral therapy, as long as the patient wishes to be treated and is willing and able to adhere to close monitoring during treatment.
Transmission risk: Transmission of HCV is primarily through exposure to infected blood. Counseling should include discussions about the specific routes of HCV transmission and advice on measures to decrease the risk of transmission to other individuals. Women of childbearing age may also be concerned about the risk of perinatal transmission.
Diet and behaviors: Patients should be informed about the natural history of HCV infection and counseled on potentially modifiable factors that are associated with accelerated liver disease, including alcohol use, obesity and insulin resistance, and marijuana use.
Because of the association with more rapid progression of liver disease, it is suggested to complete avoidance of alcohol for HCV-infected individuals. In addition, advice obese patient for weight loss and cessation of cigarettes and marijuana.
Two to three cups of coffee daily can be beneficial to liver health. Coffee consumption (more than two cups per day) has been associated with a reduced risk of hospitalization and mortality from a number of chronic liver diseases including chronic viral hepatitis, nonalcoholic steatohepatitis (NASH), and alcoholic liver disease.
Patients with hepatitis C virus (HCV) infection should be advised to abstain from alcohol use, as it accelerates the onset of cirrhosis and end-stage liver disease. Patients should be informed about the low but present risk for transmission to sex partners. Optimally, patients should use barrier protection during sexual intercourse. Sharing personal items that might have blood on them, such as toothbrushes or razors, should be avoided.
Patients with hepatitis C should not donate blood or organs. One exception is in patients with HCV who require liver transplantation.
Patients should also check with a healthcare professional before taking any new prescription pills, over-the counter drugs, or supplements, as these can potentially damage the liver.
PRECAUTIONS
Currently, no products are available to prevent hepatitis C virus (HCV) infection. The development of immunoprophylaxis for this disease is proving difficult; an effective neutralizing immune response has not been demonstrated.
- Patients with hepatitis C should be advised to abstain from alcohol use;
- They should also be advised to use barrier protection during sexual intercourse.
- Screening high-risk patients and initiating appropriate treatment may decrease the prevalence of cirrhosis and hepatocellular carcinoma (HCC).
In an ongoing prospective study of prevention of HCV infection in injection-drug users, researchers recommended six measures that can be used to prevent the spread of hepatitis C:(92)
- Reducing risk from shared ancillary drug preparation equipment, such as containers, rinse water, and filters, in addition to shared syringes
- Using a new rapid test at the point of care that offers results in 20 minutes; it can detect infection before seroconversion occurs and, combined with counseling, can help to stem transmission
- Addressing social and relational contexts of injecting can encourage uninfected individuals to take precautions when injecting drugs with infected sex partners
- Encouraging «taking a break» from injecting drugs
- Developing models to guide delivery of new prevention strategies, including already-available approaches such as increasing syringe availability and future strategies such as direct-acting antivirals that can be used prophylactically, as well as vaccines
- Combining interventions in synergistic ways, such as needle exchange and methadone maintenance programs
REFERENCES
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013; 57:1333.
- Qureshi H, Bile KM, Jooma R, Alam SE, Afridi HUR. 2010 Prevalence of hepatitis B and C viral infections in Pakistan: findings of a national survey appealing for effective prevention and control measures. East Mediterr. Health J. 16, S15–S23
- Hamid S et al. J Pak Med Assoc 2004; 54: 146-150
- Gower et al. J Hepatol. 2014;61(1 Suppl):S45-57.
- Vinod K Dhawan. Hepatitis B [Internet]. Medscape; 2018 [cited 2018 November 22]. Available from: https://emedicine.medscape.com/article/177792-overview#a3
- Ghany MG, Liang TJ. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013 Aug 15. 369 (7):679-80
- Bonkovsky HL, Mehta S. Hepatitis C: a review and update. J Am Acad Dermatol. 2001 Feb. 44(2):159-82
- Aaron S, McMahon JM, Milano D, et al. Intranasal transmission of hepatitis C virus: virological and clinical evidence. Clin Infect Dis 2008; 47:931
- Alter HJ, Purcell RH, Shih JW, et al. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med 1989; 321:1494
- Pomper GJ, Wu Y, Snyder EL. Risks of transfusion-transmitted infections: 2003. Curr Opin Hematol 2003; 10:412
- Martínez-Bauer E, Forns X, Armelles M, et al. Hospital admission is a relevant source of hepatitis C virus acquisition in Spain. J Hepatol 2008; 48:20.
- Alter MJ. Healthcare should not be a vehicle for transmission of hepatitis C virus. J Hepatol 2008; 48:2.
- Perz JF, Grytdal S, Beck S, et al. Case-control study of hepatitis B and hepatitis C in older adults: Do healthcare exposures contribute to burden of new infections? Hepatology 2013; 57:917.
- Pereira BJ, Milford EL, Kirkman RL, Levey AS. Transmission of hepatitis C virus by organ transplantation. N Engl J Med 1991; 325:454.
- Pfau PR, Rho R, DeNofrio D, et al. Hepatitis C transmission and infection by orthotopic heart transplantation. J Heart Lung Transplant 2000; 19:350.
- Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int 1994; 45:238.
- Everhart JE, Di Bisceglie AM, Murray LM, et al. Risk for non-A, non-B (type C) hepatitis through sexual or household contact with chronic carriers. Ann Intern Med 1990; 112:544.
- Dienstag JL. Sexual and perinatal transmission of hepatitis C. Hepatology 1997; 26:66S.
- Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology 2013; 57:881.
- Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology 2010; 52:1497.
- Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999; 341:556.
- Lissen E, Alter HJ, Abad MA, et al. Hepatitis C virus infection among sexually promiscuous groups and the heterosexual partners of hepatitis C virus infected index cases. Eur J Clin Microbiol Infect Dis 1993; 12:827.
- Ohto H, Terazawa S, Sasaki N, et al. Transmission of hepatitis C virus from mothers to infants. The Vertical Transmission of Hepatitis C Virus Collaborative Study Group. N Engl J Med 1994; 330:744
- Zanetti AR, Tanzi E, Paccagnini S, et al. Mother-to-infant transmission of hepatitis C virus. Lombardy Study Group on Vertical HCV Transmission. Lancet 1995; 345:289
- Resti M, Azzari C, Mannelli F, et al. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV-1. Tuscany Study Group on Hepatitis C Virus Infection. BMJ 1998; 317:437
- Fabrizi F, Messa P. Transmission of hepatitis C virus in dialysis units: a systematic review of reports on outbreaks. Int J Artif Organs 2015; 38:471
- Haley RW, Fischer RP. Commercial tattooing as a potentially important source of hepatitis C infection. Clinical epidemiology of 626 consecutive patients unaware of their hepatitis C serologic status. Medicine (Baltimore) 2001; 80:134.
- Parés A, Barrera JM, Caballería J, et al. Hepatitis C virus antibodies in chronic alcoholic patients: association with severity of liver injury. Hepatology 1990; 12:1295.
- Mendenhall CL, Moritz T, Rouster S, et al. Epidemiology of hepatitis C among veterans with alcoholic liver disease. The VA Cooperative Study Group 275. Am J Gastroenterol 1993; 88:1022.
- Koff RS, Dienstag JL. Extrahepatic manifestations of hepatitis C and the association with alcoholic liver disease. Semin Liver Dis 1995; 15:101.
- Grebely J, Page K, Sacks-Davis R, et al. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology 2014; 59:109
- Alter MJ, Margolis HS, Krawczynski K, et al. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med 1992; 327:1899
- Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008; 48:418
- Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med 1995; 332:1463
- Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med 2000; 132:296.
- Sangiovanni A, Prati GM, Fasani P, et al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology 2006; 43:1303.
- Seeff LB, Buskell-Bales Z, Wright EC, et al. Long-term mortality after transfusion-associated non-A, non-B hepatitis. The National Heart, Lung, and Blood Institute Study Group. N Engl J Med 1992; 327:1906.
- Seeff LB, Hollinger FB, Alter HJ, et al. Long-term mortality and morbidity of transfusion-associated non-A, non-B, and type C hepatitis: A National Heart, Lung, and Blood Institute collaborative study. Hepatology 2001; 33:455.
- Kiyosawa K, Sodeyama T, Tanaka E, et al. Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: analysis by detection of antibody to hepatitis C virus. Hepatology 1990; 12:671.
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349:825.
- Zarski JP, Mc Hutchison J, Bronowicki JP, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol 2003; 38:307.
- Barrett S, Goh J, Coughlan B, et al. The natural course of hepatitis C virus infection after 22 years in a unique homogenous cohort: spontaneous viral clearance and chronic HCV infection. Gut 2001; 49:423.
- Datz C, Cramp M, Haas T, et al. The natural course of hepatitis C virus infection 18 years after an epidemic outbreak of non-A, non-B hepatitis in a plasmapheresis centre. Gut 1999; 44:563.
- Seeff LB, Miller RN, Rabkin CS, et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med 2000; 132:105.
- Wiese M, Berr F, Lafrenz M, et al. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single-source outbreak in germany: a 20-year multicenter study. Hepatology 2000; 32:91.
- Rodger AJ, Roberts S, Lanigan A, et al. Assessment of long-term outcomes of community-acquired hepatitis C infection in a cohort with sera stored from 1971 to 1975. Hepatology 2000; 32:582.
- de Lédinghen V, Trimoulet P, Mannant PR, et al. Outbreak of hepatitis C virus infection during sclerotherapy of varicose veins: long-term follow-up of 196 patients (4535 patient-years). J Hepatol 2007; 46:19.
- Kielland KB, Delaveris GJ, Rogde S, et al. Liver fibrosis progression at autopsy in injecting drug users infected by hepatitis C: a longitudinal long-term cohort study. J Hepatol 2014; 60:260.
- Fransen van de Putte DE, Makris M, Fischer K, et al. Long-term follow-up of hepatitis C infection in a large cohort of patients with inherited bleeding disorders. J Hepatol 2014; 60:39.
- Butt AA, Yan P, Lo Re V 3rd, et al. Liver fibrosis progression in hepatitis C virus infection after seroconversion. JAMA Intern Med 2015; 175:178.
- Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology 1997; 112:463
- Hu KQ, Tong MJ. The long-term outcomes of patients with compensated hepatitis C virus-related cirrhosis and history of parenteral exposure in the United States. Hepatology 1999; 29:1311
- Planas R, Ballesté B, Alvarez MA, et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatol 2004; 40:823
- Bruno S, Crosignani A, Maisonneuve P, et al. Hepatitis C virus genotype 1b as a major risk factor associated with hepatocellular carcinoma in patients with cirrhosis: a seventeen-year prospective cohort study. Hepatology 2007; 46:1350
- Sanjiv Chopra. Clinical manifestations and natural history of chronic hepatitis C virus infection [Internet]. Uptodate; 2018 [cited 2018 November 22]. Available from: https://www.uptodate.com/contents/clinical-manifestations-and-natural-history-of-chronic-hepatitis-c-virus-infection?topicRef=3675&source=see_link
- Evon DM, Stewart PW, Amador J, et al. A comprehensive assessment of patient reported symptom burden, medical comorbidities, and functional well being in patients initiating direct acting antiviral therapy for chronic hepatitis C: Results from a large US multi-center observational study. PLoS One 2018; 13:e0196908.
- Spiegel BM, Younossi ZM, Hays RD, et al. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology 2005; 41:790
- Rodger AJ, Jolley D, Thompson SC, et al. The impact of diagnosis of hepatitis C virus on quality of life. Hepatology 1999; 30:1299
- Ware JE Jr, Bayliss MS, Mannocchia M, Davis GL. Health-related quality of life in chronic hepatitis C: impact of disease and treatment response. The Interventional Therapy Group. Hepatology 1999; 30:550
- Forton DM, Allsop JM, Main J, et al. Evidence for a cerebral effect of the hepatitis C virus. Lancet 2001; 358:38.
- Forton DM, Thomas HC, Murphy CA, et al. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology 2002; 35:433.
- Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology 2002; 35:440.
- Kramer L, Bauer E, Funk G, et al. Subclinical impairment of brain function in chronic hepatitis C infection. J Hepatol 2002; 37:349
- Conry-Cantilena C, VanRaden M, Gibble J, et al. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med 1996; 334:1691.
- Barreiro P, Labarga P, Fernández-Montero JV, et al. Longitudinal changes in viral RNA concentration in patients with chronic hepatitis C and/or HIV infection in the absence of antiviral therapy. J Clin Virol 2013; 58:391
- Fanning L, Kenny-Walsh E, Levis J, et al. Natural fluctuations of hepatitis C viral load in a homogeneous patient population: a prospective study. Hepatology 2000; 31:225.
- Arase Y, Ikeda K, Chayama K, et al. Fluctuation patterns of HCV-RNA serum level in patients with chronic hepatitis C. J Gastroenterol 2000; 35:221
- Anand BS, Velez M. Assessment of correlation between serum titers of hepatitis C virus and severity of liver disease. World J Gastroenterol 2004; 10:2409.
- Nguyen TT, Sedghi-Vaziri A, Wilkes LB, et al. Fluctuations in viral load (HCV RNA) are relatively insignificant in untreated patients with chronic HCV infection. J Viral Hepat 1996; 3:75.
- Kirk GD, Mehta SH, Astemborski J, et al. HIV, age, and the severity of hepatitis C virus-related liver disease: a cohort study. Ann Intern Med 2013; 158:658.
- Daar ES, Lynn H, Donfield S, et al. Relation between HIV-1 and hepatitis C viral load in patients with hemophilia. J Acquir Immune Defic Syndr 2001; 26:466.
- Thomas DL, Shih JW, Alter HJ, et al. Effect of human immunodeficiency virus on hepatitis C virus infection among injecting drug users. J Infect Dis 1996; 174:690.
- Sagnelli E, Coppola N, Messina V, et al. HBV superinfection in hepatitis C virus chronic carriers, viral interaction, and clinical course. Hepatology 2002; 36:1285.
- García-Suárez J, Burgaleta C, Hernanz N, et al. HCV-associated thrombocytopenia: clinical characteristics and platelet response after recombinant alpha2b-interferon therapy. Br J Haematol 2000; 110:98
- Adinolfi LE, Giordano MG, Andreana A, et al. Hepatic fibrosis plays a central role in the pathogenesis of thrombocytopenia in patients with chronic viral hepatitis. Br J Haematol 2001; 113:590
- Merican I, Sherlock S, McIntyre N, Dusheiko GM. Clinical, biochemical and histological features in 102 patients with chronic hepatitis C virus infection. Q J Med 1993; 86:119
- Recommendations for Testing, Managing, and Treating Hepatitis C. Joint panel from the American Association of the Study of Liver Diseases and the Infectious Diseases Society of America. http://www.hcvguidelines.org/ (Accessed on November 23, 2018).
- Pereira BJ, Levey AS. Hepatitis C virus infection in dialysis and renal transplantation. Kidney Int 1997; 51:981
- Lau JY, Davis GL, Brunson ME, et al. Hepatitis C virus infection in kidney transplant recipients. Hepatology 1993; 18:1027
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO Hepatitis C in CKD Guideline: welcoming advances in evaluation and management. Kidney Int 2018; 94:663
- Seeff LB. Natural history of chronic hepatitis C. Hepatology 2002; 36:S35
- Centers for Disease Control and Prevention (CDC). Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep 2013; 62:362
- Stockman LJ, Guilfoye SM, Benoit AL, et al. Rapid hepatitis C testing among persons at increased risk for infection–Wisconsin, 2012-2013. MMWR Morb Mortal Wkly Rep 2014; 63:309
- Freiman JM, Tran TM, Schumacher SG, et al. Hepatitis C Core Antigen Testing for Diagnosis of Hepatitis C Virus Infection: A Systematic Review and Meta-analysis. Ann Intern Med 2016; 165:345
- Khan H, Hill A, Main J, et al. Can Hepatitis C Virus Antigen Testing Replace Ribonucleic Acid Polymearse Chain Reaction Analysis for Detecting Hepatitis C Virus? A Systematic Review. Open Forum Infect Dis 2017; 4:ofw252
- World Health Organization. WHO Guidelines on Hepatitis B and C Testing, 2017. http://apps.who.int/iris/bitstream/10665/254621/1/9789241549981-eng.pdf?ua=1 (Accessed on November 23, 2018).
- Everson GT, Weinberg H. Living With Hepatitis C: A Survivor’s Guide. 3rd ed. Long Island City, New York: Hatherleigh Health; 2002.
- World Health Organization. Hepatitis C: fact sheet. Available at http://www.who.int/mediacentre/factsheets/fs164/en/. Updated: October 2017; Accessed: January 23, 2018
- [Guideline] American Association for the Study of Liver Diseases (AASLD)/Infectious Diseases Society of America (IDSA). Recommendations for testing, managing, and treating hepatitis C. Available at http://www.hcvguidelines.org/full-report-view. Updated: September 21, 2017; Accessed: January 23, 2018.
- Pockros PJ. New direct-acting antivirals in the development for hepatitis C virus infection. Therap Adv Gastroenterol 2010; 3:191.
- Prescribing Information for OLYSIOTM (simeprevir): Janssen Therapeutics, Division of Janssen Products, LP, Titusville NJ 08560. Issued November 2013.
- Page K, Morris MD, Hahn JA, Maher L, Prins M. Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin Infect Dis. 2013 Aug. 57 suppl 2:S32-8.
