EPIDEOMOLOGY
Fever developing during neutropenia is a frequent complication in neutropenic cancer patients, affecting 80% of those with hematological malignancies and 10–50% of those with solid malignancies.(1,2) Its incidence in solid metastatic cancers was estimated to be about 13–21%, depending on the underlying disease, and occurred mostly during the first chemotherapy cycle.(3) The possibility of bloodstream infections (BSIs) is the major concern at the onset of fever, since it accounts for 10–25% of all febrile episodes in neutropenic patients, with an incidence as high as 13–60% in haematopoietic stem cell transplantation (HSCT) recipients.(1,4) Moreover, the occurrences of severe sepsis and septic shock in the setting of febrile neutropenia have been estimated to be, respectively, 20–30% and 5–10%.(5-7)
PATHOPHYSIOLOGY
Pathophysiology of CIN is as follows:
- Decreased production of neutrophils from the bone marrow.(8)
- Shift of circulating neutrophils to the vascular endothelium or tissues such as the spleen, termed “margination”, which can occur with splenomegaly and/or hypersplenism.(8)
- Immune-mediated destruction: The current hypothesis of immune-mediated drug-induced agranulocytosis suggests that the drug, or more commonly a reactive metabolite of the drug, irreversibly binds to the neutrophil membrane. In some cases, the reactive metabolite results in the production of antibodies or T cells directed against the altered membrane structure; in others, true anti-neutrophil autoantibodies are produced that do not require the presence of the drug .(9)
- Direct toxic effects upon marrow granulocytic precursors: Some drugs can directly damage myeloid precursors. As an example, detoxification of many non-polar compounds requires conversion to a chemically reactive intermediate that may bind to nuclear material or cytoplasmic proteins, causing direct toxicity.(10)
Definition
Neutropenia: Neutropenia is defined as an ANC of <500 cells/mm3 or an ANC that is expected to decrease to <500 cells/mm3 during the next 48 h.(11)
Fever: Fever is defined as a single oral temperature measurement of ≥38.3oC (101oF) or a temperature of ≥38.0oC (100.4oF) sustained over a 1-h period.(11)
Categories of neutropenia
Neutropenia can be categorized as mild, moderate, or severe:(12)
- Mild neutropenia – Absolute neutrophil count >1000 and <1500 cells/microL
- Moderate neutropenia – Absolute neutrophil count >500 and <1000cells/microL
- Severe neutropenia – Absolute neutrophil count<500 cells/microL
Grading of neutropenia
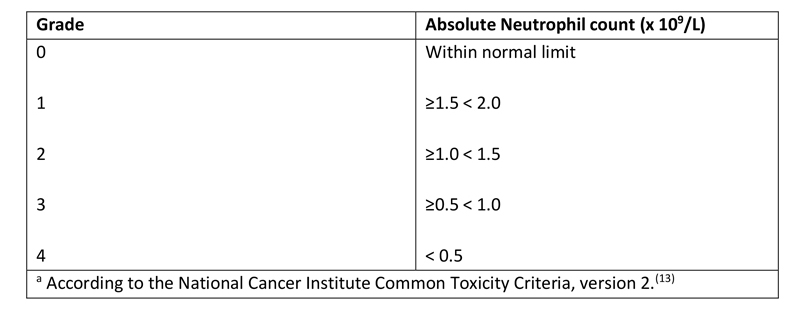
The Common Toxicity Criteria of the National Cancer Institute is the most commonly used scale for grading the severity of the cytopenias associated with cancer chemotherapy; it delineates neutropenia into 4 grades (Table 1).(13)
Table 1 - Grades of Neutropeniaa
SIGNS AND SYMPTOMS
Patients with cancer undergoing chemotherapy, especially if neutropenic, are highly susceptible to almost any type of bacterial or fungal infection. Recurrent infections are commonly seen with neutropenia. The most common site of infection is the oral cavity and mucous membranes, presenting as oral ulcers, pharyngitis, and periodontitis. Skin is another site of infection, with rashes, ulcerations, abscesses, and poor wound healing. Perirectal and genital infections are also common. Systemic infections of the lungs, gastrointestinal tract, and blood stream occur and may prove fatal in patients with persistent severe neutropenia.(14)
Symptoms that may accompany CIN include:(15)
- A fever, which is a temperature of 100.5°F or higher
- Chills or sweating
- Sore throat, sores in the mouth, or a toothache
- Abdominal pain
- Pain near the anus
- Pain or burning when urinating, or urinating often
- Diarrhea or sores around the anus
- A cough or shortness of breath
- Any redness, swelling, or pain, particularly around a cut, wound, or where a catheter was placed
Unusual vaginal discharge or itching
DIAGNOSTIC TESTS(16)
In all cancer patients presenting with neutropenic fever, empiric antibacterial therapy should be initiated immediately after blood cultures have been obtained and before any other investigations have been completed.
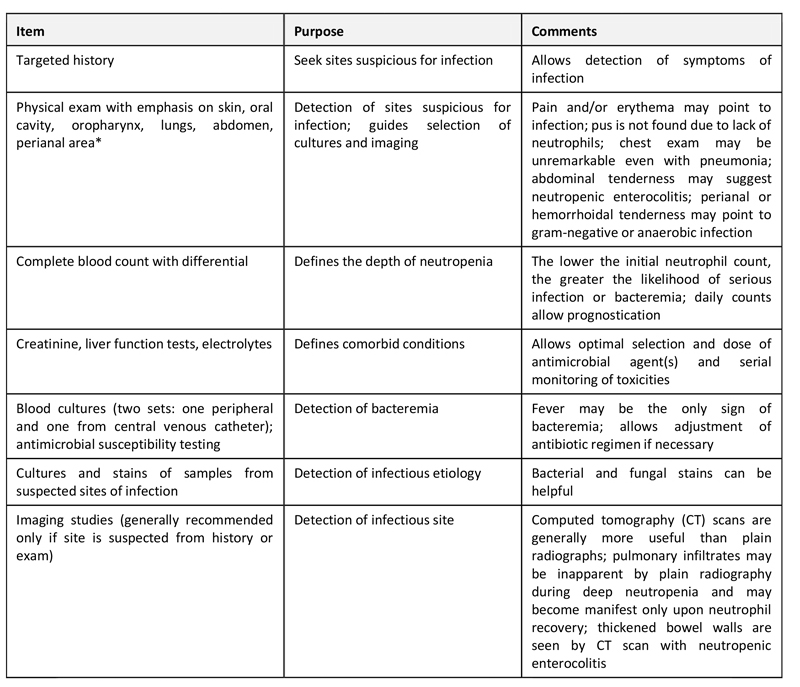
All patients should undergo a careful history and detailed physical examination as well as laboratory, microbiology, and imaging studies; the approach to the diagnostic evaluation is summarized in the following table (table 1). Because symptoms and signs of infection are attenuated due to the lack of an inflammatory reaction, fever may be the sole sign of infection. Thus, it is important to recognize that the absence of the typical symptoms, signs, or laboratory findings suggestive of infection typically seen in non-neutropenic patients cannot be used to exclude the possibility of infection. The evaluation should be performed promptly.
Table 1: Diagnostic evaluation of patients with fever and neutropenia
* Digital rectal examination is avoided in neutropenic patients.
History: The history should probe for the development of new symptoms that can lead to identification of a site of infection. The initial clinical evaluation also focuses on assessing the risk of serious complications.
The following key elements of the history should always be included:
- Inquire about organ-specific symptoms: A review of systems should be repeated daily. Determine if antimicrobial prophylaxis had been given and which agent(s).
- Probe for a history of prior infection or colonization (especially by antibiotic-resistant organisms).
- Determine if there might be noninfectious causes of fever (e.g. blood transfusion, uncontrolled cancer).
- Determine which comorbid conditions may be present: A variety of comorbid conditions predispose to infectious complications, such as immobility (decubitus ulcers), poor nutrition, foreign bodies such as venous or bladder catheters, prosthetic heart valves or orthopedic hardware, diabetes mellitus, chronic respiratory conditions, rheumatologic disorders, inflammatory bowel disease, etc.
Risk assessment: The initial clinical evaluation focuses on assessing the risk of serious complications. This risk assessment dictates the approach to therapy, including the need for inpatient admission, intravenous (IV) antibiotics, and prolonged hospitalization.
Physical examination: A thorough general physical examination should be performed. The emphasis should be on sites most likely to be infected, including the skin, catheter sites, biopsy and bone marrow aspirate sites, teeth, oropharynx and gingival surfaces, sinuses, lungs, abdomen, genitals, and perianal area. As noted above, it is important to remember that in the absence of neutrophils, signs of inflammation can be extremely subtle.
Review of systems and a physical examination should be repeated daily. In patients with persistent fever, new sites of infection (e.g. lungs, skin, and urinary tract) may become apparent over time. In addition, as the neutrophil count recovers, localizing symptoms and signs of infection often become evident for the first time.
Lungs: The lungs are a common site of infection in patients with chemotherapy-induced neutropenia and should be examined for signs of pneumonia (e.g. rales). Hypoxia, tachypnea, and increased work of breathing are other signs of pneumonia.
Abdomen: An abdominal examination should be performed to evaluate for peritoneal signs and/or abdominal tenderness, which may represent neutropenic enterocolitis or Clostridioides (formerly Clostridium) difficile colitis. Even when an abdominal process is present, abdominal signs may be subtle or absent in neutropenic patients.
Intravenous catheter sites: All IV catheter sites, especially central venous catheter (CVC) sites, should be carefully examined for subtle signs of infection; slight erythema or tenderness may be the only evidence of a serious “tunnel” infection. Fluctuance or exudates are unlikely since the lack of inflammatory cells inhibits the development of inflammatory reactions. IV catheters should also be assessed for any malfunction; difficulty with infusion or blood drawing can also be a sign of an infected venous thrombosis, even in the absence of a problem with the exit site. Sites of recent IV catheters should also be examined.
Skin and mucous membranes: The skin and mucous membranes should be examined for signs of erythema, rash, cellulitis, ulcers, furuncles, vesicles, paronychia, mucositis, dental or peritonsillar cellulitis, perianal fissures, and pilonidal disease. Skin lesions can often be a manifestation of a systemic infection including:
- Ulcers – Fungi, atypical bacteria, nontuberculous mycobacteria, viruses
- Vesicles – Viruses
- Nodules – Fungi, bacteria, nontuberculous mycobacteria
- Ecthyma gangrenosum – Large lesion with a necrotic center that is classically seen with Pseudomonas aeruginosa but also with other bacteria, such as Staphylococcus aureus
- Skin lesions with a necrotic center – Invasive fungal infections (e.g. Fusarium spp, Aspergillus spp, Mucorales) can also cause skin lesions with a necrotic center
Sometimes, skin lesions, such as erythema multiforme, can be related to viral infections; alternatively, erythema multiforme can be related to antibiotic therapy and may be associated with fever, causing diagnostic confusion.
Perianal region: The examination should also include inspection of the perianal area. Erythema, pain on palpation, and tender hemorrhoids are important signs of infection. However, digital rectal examination (and rectal temperatures) should be avoided so that one does not introduce infection by traumatizing the fragile mucosa.
Routine laboratory tests: Laboratory evaluation should include a complete blood cell count with differential, hepatic transaminases, bilirubin, electrolytes, serum creatinine, blood urea nitrogen, serum lactate, urinalysis, and cultures (table 1).
In interpreting laboratory results in neutropenic patients, it is important to recognize that the absence of the typical laboratory findings suggestive of infection that are usually seen in non-neutropenic patients cannot be used to exclude the possibility of infection. Therefore, absence of abnormalities, such as cerebrospinal fluid (CSF) pleocytosis, pyuria, or neutrophils on sputum Gram stain, does not rule out infection.
Serum fungal markers: Checking fungal markers from the serum, such as the Aspergillus galactomannan antigen and the beta-D-glucan assay, should also be considered in high-risk patients. Fungal markers are checked starting with onset of neutropenia and continued until neutrophil recovery. They are not routinely sent in low-risk patients except as part of an evaluation for suspected fungal infection. Some centers perform serial monitoring of these twice weekly in hematopoietic cell transplant recipients and acute leukemia patients not receiving anti-mold prophylaxis, where a positive test may guide further evaluation or prompt pre-emptive therapy. In patients receiving an agent with antimold activity, the value of this approach has not been established. The Aspergillus galactomannan antigen is a specific test for invasive aspergillosis, whereas the beta-D-glucan assay is a nonspecific test for several invasive fungal infections, including aspergillosis and candidiasis.;
Microbiology and other diagnostic testingIn all cancer patients presenting with neutropenic fever, empiric antibacterial therapy should be initiated immediately after blood cultures have been obtained and before any other investigations have been completed. The microbiologic evaluation should be performed promptly.
Specimens for the microbiology laboratory should include at least two sets of blood cultures (table 1). Each set should have a volume of 20 mL (proportionally smaller volume in children weighing <40 kg; e.g. no more than 1 percent of estimated patient total blood volume). In patients with a CVC, it is preferred to collect a set of blood cultures simultaneously from each lumen and from a peripheral vein site. Some experts feel that it is acceptable to collect both sets of cultures from the CVC, especially in patients with difficult peripheral venous access. In patients without a CVC, a set should be obtained from each of two separate venipuncture sites.
Two sets of blood cultures are typically repeated daily for persistent fevers or rigors at least during the first two days following initiation of empiric antibiotics. Institutions may have different guidelines for the frequency of obtaining blood cultures. As an example, some centers stop collecting blood cultures or reduce the frequency of collection after several days if the initial cultures are negative for patients with a stable fever pattern. If the cultures are positive, they should be repeated until they become negative to ensure response to therapy and intermittently thereafter.
Specimens should be obtained from other sites as clinically indicated (e.g. sputum, urine, CVC exit site, CSF, skin, stool). Many neutropenic patients receiving cytotoxic chemotherapy are also thrombocytopenic; in such patients, platelet transfusions are often necessary in order to safely perform a lumbar puncture or other invasive procedures. In general, indications for collection of these additional samples are as follows:
- Stool: If diarrhea is present, send a C. difficile toxin assay.
- Urine: If symptoms of urinary tract infection are present, send urine for bacterial culture and susceptibility testing.
- Skin: If skin lesions are present, aspirate or biopsy lesions for bacterial and fungal stains and cultures, and, if vesicles are present, send for either herpes simplex virus and varicella-zoster virus polymerase chain reactions (PCRs) or culture plus direct fluorescent antibody testing. When a biopsy is obtained, it should also be sent for histopathology.
- Respiratory: If productive cough is present, send sputum for bacterial and fungal stains and cultures and assays for respiratory viruses.
Neutropenic patients with pulmonary infiltrates often cannot produce sputum; a more invasive approach, including bronchoscopy with bronchoalveolar lavage or video-assisted thoracoscopic surgery, may need to be pursued in order to make a microbiologic diagnosis. This may be particularly important for patients with infiltrates on chest radiographs or chest computed tomography (CT) who continues to worsen despite 24 to 48 hours of empiric antibiotic therapy.
In patients with an influenza-like illness (especially when respiratory viruses are circulating), a nasopharyngeal swab should be sent for influenza testing (ideally with a molecular test, such as PCR), as well as testing for other respiratory viruses (respiratory syncytial virus, adenovirus, parainfluenza virus, human metapneumovirus).
- Cerebrospinal fluid: If symptoms of meningitis (fever, headache, nuchal rigidity, altered mental status) are present, send CSF for cell count, glucose, protein, and bacterial culture and susceptibility testing. In addition, in patients with clinical signs of meningitis and/or encephalitis, CSF should also be sent for cryptococcal antigen testing and for PCR testing for herpes simplex virus, cytomegalovirus, varicella-zoster virus, and, in allogeneic hematopoietic cell transplant recipients, human herpesvirus 6. Additional tests are indicated if epidemiologic risk factors are present (e.g. testing for West Nile virus, Eastern equine encephalitis, and other arthropod-borne encephalitides during the summer in certain regions).
- Imaging: Imaging studies should be performed promptly but should not delay initiation of empiric therapy.
In febrile neutropenic patients with respiratory signs or symptoms, it is suggested that a chest radiograph be obtained in low-risk patients and a noncontrast intermediate- or high-resolution chest CT be obtained in high-risk patients. In contrast, the Infectious Diseases Society of America guidelines recommend a chest radiograph as part of the initial evaluation for neutropenic fever in patients with respiratory symptoms without distinction by risk status. It is important to note, however, that chest radiograph findings are often minimal or absent, even in patients with pneumonia or pulmonary nodules. Intermediate- or high-resolution chest CT is much more sensitive for detecting abnormalities in neutropenic patients. One should be mindful that detection of small nodules may represent benign granulomas in certain areas endemic for certain infections rather than active infection, and therefore caution in interpretation should be exercised.
CT scanning of other sites (head, sinuses, abdomen/pelvis) should be performed according to suggestive symptoms or other risk factors. In particular, patients with neutropenic fever and any signs or symptoms suggestive of neutropenic enterocolitis or C. difficile colitis (e.g. abdominal pain or tenderness, diarrhea) should undergo abdominal CT scanning with IV and oral contrast.
THERAPY CONSIDERATION
Approaches to the management of infection in patients at risk for neutropenic fever include primary prophylaxis, secondary prophylaxis, empiric therapy, and preemptive therapy.
Primary prophylaxis: Primary prophylaxis involves the administration of an antimicrobial drug to prevent infection in patients at increased risk.
Secondary prophylaxis: Secondary prophylaxis involves the administration of prophylactic doses of an antimicrobial drug to prevent recurrent infection.
Empiric therapy: In patients with chemotherapy-induced neutropenia, empiric therapy involves the initiation of therapy at the time of the onset of neutropenic fever but before a firm diagnosis of infection has been established. Empiric antimicrobial therapy is a standard part of the management of neutropenic fever.
Preemptive therapy: Preemptive therapy involves the initiation of therapy based upon screening with a sensitive microbiology assay (e.g. antigen detection or molecular assays) in an attempt to detect the presence of a putative pathogen or early subclinical infection. Patients whose infections are detected using a preemptive approach are treated to avoid progression to invasive disease. A preemptive approach is sometimes used for antifungal therapy.
TREATMENT OPTIONS
Antibiotic therapy:(17)
Fever in a neutropenic patient should be considered a medical emergency. Broad-spectrum antibacterials should be given as soon as possible (within 60 minutes of triage) and at full doses, adjusted for renal and/or hepatic function. In addition, the diagnostic evaluation should be obtained quickly.
The aim of empiric therapy is to cover the most likely and most virulent pathogens that may rapidly cause serious or life-threatening infection in neutropenic patients. The following general principles apply:
- Antibiotics are usually administered empirically but should always include appropriate coverage for suspected or known infections. Even when the pathogen is known, the antibiotic regimen should provide broad-spectrum empiric coverage for the possibility of other pathogens, unlike the treatment strategy adopted in many immunocompetent hosts.
- In high-risk patients, antibiotics should generally be administered intravenously (IV) in a hospital setting.
- Initial antibiotic selection should be guided by the patient’s history, allergies, symptoms, signs, recent antibiotic use and culture data, and awareness of the susceptibility patterns of institutional nosocomial pathogens.
- Ideally, antibiotics should be bactericidal.
- Clinical response and culture and susceptibility results should be monitored closely, and therapy should be adjusted in a timely fashion in response to this information.
Febrile neutropenic patients should be monitored frequently with respect to vital signs (blood pressure, heart rate, respiratory rate, and temperature), performance status (the clinical burden of the neutropenic fever syndrome), and the ability to achieve adequate oral intake in the presence of oral or gastrointestinal mucositis. Temporarily holding administration of systemic chemotherapy should be considered during the management of the sepsis syndrome until the patient stabilizes. Attention to fluid and electrolyte management is important given the dehydrating effects of fever, vomiting, and/or diarrhea. Urine output of >0.5 mL/kg per hour should be maintained.
Afebrile neutropenic patients with new signs or symptoms that are consistent with infection should be evaluated and managed as if they are febrile.
Timing of antibiotics: In all febrile neutropenic patients, empiric broad-spectrum antibacterial therapy should be initiated immediately after blood cultures have been obtained and before any other investigations have been completed. Antimicrobial therapy should be administered within 60 minutes of presentation.
Initial regimen: The choice of antibiotics is driven by multiple factors, including the degree of immunocompromise, prior antibiotic and infection history, local patterns of antibiotic resistance, and whether an agent is bactericidal or not. Some drugs, such as beta-lactams, exhibit time-dependent killing. When using beta-lactams, correct dosing intervals should be used to ensure that drug concentrations are greater than the minimal inhibitory concentration for the pathogen. There has been increasing interest in giving prolonged infusions of beta-lactams (either an extended infusion over three or four hours or a continuous infusion) rather than traditional dosing over 30 minutes to optimize pharmacodynamics. Indications for the use of prolonged infusion strategies with beta-lactams are not established, but it is reasonable to give prolonged infusions in patients with neutropenic fever who are severely ill and/or who have an elevated risk of drug-resistant gram-negative bacilli.
Other antibiotics, such as aminoglycosides and fluoroquinolones, exhibit concentration-dependent killing and are important in the treatment of gram-negative sepsis.
The Infectious Diseases Society of America (IDSA) recommends the following approach for the initial therapy of high-risk neutropenic patients with fever:(1)
Initiation of monotherapy with an antipseudomonal beta-lactam agent, such as cefepime, meropenem, imipenem-cilastatin, or piperacillin-tazobactam. Ceftazidime monotherapy has also been shown to be effective and continues to be used at some cancer centers with low rates of resistance. Ceftazidime monotherapy should not be used when there is concern for a gram-positive infection, such as an infection caused by viridans group streptococci in patients with severe mucositis. The dosing of these agents for patients with normal renal function using traditional dosing (i.e. over 30 minutes) are:
- Cefepime – 2 g IV every eight hours
- Meropenem – 1 g IV every eight hours
- Imipenem-cilastatin – 500 mg IV every six hours
- Piperacillin-tazobactam – 4.5 g IV every six to eight hours; if there is significant concern for Pseudomonas infection (particularly in those who are severely ill or were not receiving fluoroquinolone prophylaxis at the time of onset of illness), 4.5 g IV every six hours should be given
- Ceftazidime – 2 g IV every eight hours
As noted above, prolonged infusions of beta-lactams can be used instead of traditional dosing when optimization of pharmacodynamics is thought to be important.
- Other antibiotics (e.g. aminoglycosides, fluoroquinolones, and/or vancomycin) may be added to the initial regimen in patients with complicated presentations (e.g. hypotension and/or mental status changes), focal findings (e.g. pneumonia or cellulitis), or if antimicrobial resistance is suspected or proven.
- Vancomycin (or other agents that target gram-positive cocci) is not recommended as a standard part of the initial regimen but should be added in certain patients, such as those with suspected catheter-related infection, skin or soft tissue infection, pneumonia, or hemodynamic instability.
- Modifications to the initial regimen should be considered for patients at risk for infection with antibiotic-resistant organisms, patients who are clinically unstable, and patients with positive blood cultures that are suggestive of a resistant infection. Risk factors for infections caused by resistant bacteria include previous infection or colonization by the organism and/or treatment in a hospital with high rates of resistance.
Comparisons of regimens
Monotherapy: Monotherapy with a beta-lactam agent with activity against Pseudomonas aeruginosa (e.g. cefepime, meropenem, imipenem-cilastatin, piperacillin-tazobactam, or ceftazidime) is frequently employed; clinical trials with ceftazidime, imipenem-cilastatin, or meropenem have demonstrated equivalent outcomes compared with two-drug regimens. In addition, fewer adverse events have generally been seen with monotherapy regimens compared with combination regimens. The majority of the regimens evaluated provided coverage targeted at gram-negative bacilli, especially P. aeruginosa.
One concern about monotherapy is the possibility that increasing rates of antibiotic resistance in a number of pathogens may reduce the efficacy of this strategy. Single agents, especially ceftazidime, may actually promote the outgrowth of resistant organisms in this group of patients who require frequent antibiotic administration. It is therefore important to maintain vigilance for the emergence of antibiotic resistance locally that may necessitate a change in antibiotic practices.
Combination therapy: Numerous combination antibiotic regimens have been studied as initial empiric therapy in neutropenic fever, but none has been shown to be clearly superior to others or to monotherapy. One approach is to use an extended-spectrum beta-lactam (e.g. piperacillin, ceftazidime) in combination with an aminoglycoside. Other examples of combination regimens include double beta-lactams or a beta-lactam and a fluoroquinolone.
Penicillin-allergic patients: Many patients with a history of allergy to penicillin tolerate cephalosporin. However, those with a history of an immediate-type hypersensitivity reaction (e.g., hives and/or bronchospasm) should not receive beta-lactams or carbapenems. Alternative empiric regimens for such patients include aztreonam plus vancomycin or ciprofloxacin plus clindamycin.(1) Of these regimens, ir is preferred aztreonam plus vancomycin out of concern for increasing the risk of Clostridioides (formerly Clostridium) difficile infection in those requiring clindamycin for an extended period. In general, fluoroquinolones should not be used in patients who have received them recently (including those receiving a fluoroquinolone for neutropenic prophylaxis). The decision of which alternative regimen to use in penicillin-allergic patients should be made based upon the susceptibility patterns of bacteria (especially gram-negative bacilli) at the individual institution as well as the patient’s previous microbiologic data.
Addition of gram-positive coverage: Routine addition of gram-positive antibiotic coverage to the initial empiric antibiotic regimen has not been associated with significant clinical benefit.
Vancomycin (or other agents that target gram-positive cocci) is not recommended as a standard part of the initial regimen, but gram-positive coverage should be added in patients with any of the following findings:(1)
- Hemodynamic instability or other signs of severe sepsis
- Pneumonia
- Positive blood cultures for gram-positive bacteria while awaiting speciation and susceptibility results
- Suspected central venous catheter (CVC)-related infection (e.g. chills or rigors during infusion through a CVC and/or cellulitis around the catheter entry site)
- Skin or soft tissue infection
- Severe mucositis in patients who were receiving prophylaxis with a fluoroquinolone lacking activity against streptococci and in whom ceftazidime is being used as empiric therapy. Addition of gram-positive coverage is recommended in this situation because of the increased risk of viridans group streptococcal infections, which can result in sepsis and the acute respiratory distress syndrome.
Empiric gram-positive coverage is particularly important for patients who are colonized with methicillin-resistant S. aureus (MRSA), vancomycin-resistant enterococci (VRE), or penicillin- or ceftriaxone-resistant streptococci who become hemodynamically unstable or develop bacteremia with gram-positive cocci.
The combination of vancomycin and piperacillin-tazobactam has been associated with acute kidney injury. In patients who require vancomycin and an antipseudomonal beta-lactam, a beta-lactam other than piperacillin-tazobactam can be used (e.g. cefepime). If vancomycin is used with piperacillin-tazobactam, renal function should be monitored carefully and the regimen should be adjusted to reduce further nephrotoxicity if acute kidney injury develops.
Daptomycin is another alternative to vancomycin, but it has been less well studied and should not be used for pulmonary infections because it is inactivated by surfactant and therefore does not achieve sufficiently high concentrations in the respiratory tract.
Antibiotic resistance: The increasing frequency of multidrug-resistant gram-negative bacterial infections is forcing the renewed use of older agents that have been used infrequently in febrile neutropenic cancer patients, such as colistin (colistimethate) and fosfomycin, and newer agents, such as tigecycline. Similarly, beta-lactam- or glycopeptide-resistant gram-positive organisms have forced the use of lipopeptides (daptomycin), oxazolidinones (linezolid), and glycylcyclines (tigecycline).
The patient’s risk for the following resistant organisms should be considered when choosing an empiric regimen:
- Among gram-positive organisms, pathogens with acquired resistance include coagulase-negative staphylococci, MRSA, VRE, and penicillin- and ceftriaxone-resistant Streptococcus pneumoniae and other streptococci.
- Gram-positive organisms that have intrinsic resistance to vancomycin (Leuconostoc, Lactobacillus, and Pediococcus spp).
- Multidrug-resistant gram-negative bacilli, such as P. aeruginosa, Escherichia coli, and Citrobacter, Acinetobacter, and Stenotrophomonas spp. The use of fluoroquinolones for prophylaxis has contributed to the emergence of antibiotic resistance.
- The presence of extended-spectrum beta-lactamases (ESBL), plasmid-mediated AmpC-type beta-lactamases, and carbapenemase-producing bacteria (e.g. Klebsiella pneumoniae carbapenemase [KPC]) can severely limit treatment options.
Strategies to reduce drug resistance include limiting prophylaxis, using targeted therapy when feasible, discontinuing unneeded empiric therapies (e.g. vancomycin) when cultures remain negative, and initiation of antibiotic stewardship programs.
The following antibiotics can be used when resistant infections are suspected:(1)
- MRSA – Vancomycin, linezolid, or daptomycin; daptomycin should be avoided in patients with pneumonia because it does not achieve sufficiently high concentrations in the respiratory tract.
- VRE – Linezolid or daptomycin.
- ESBL-producing gram-negative bacilli – A carbapenem (e.g. imipenem, meropenem).
- Carbapenemase-producing bacteria, including K. pneumoniae carbapenemase – Colistin or tigecycline.
Addition of an antifungal agent
Indications: An empiric antifungal agent should be added after four to seven days in high-risk neutropenic patients who are expected to have a total duration of neutropenia >7 days who have persistent or recurrent fever and in whom reassessment does not yield a cause. The rationale for this approach is that undiagnosed fungal infection was found in early studies in many patients who died during prolonged neutropenia. The incidence of fungal infection (especially those caused by Candida or Aspergillus spp) rises after patients have experienced more than seven days of persistent neutropenic fever.
In patients who are clinically unstable or have a suspected fungal infection, antifungal therapy should be considered even earlier than what is recommended for empiric therapy.
Choice of drug: The choice of agent for empiric antifungal therapy depends upon which fungi are most likely to be causing infection as well as the toxicity profiles and cost.(1) In patients who have not been receiving antifungal prophylaxis, Candida spp are the most likely cause of invasive fungal infection. In patients receiving fluconazole prophylaxis, fluconazole-resistant Candida spp (e.g. C. glabrata and C. krusei) and invasive mold infections, particularly Aspergillus spp, are the most likely causes.
The 2010 IDSA guidelines for empiric antifungal therapy recommend amphotericin B deoxycholate, a lipid formulation of amphotericin B, caspofungin, voriconazole, or itraconazole as suitable options for empiric antifungal therapy in neutropenic patients.(1)
Dosing: The dosing of the various antifungal agents recommended above is as follows:
- Caspofungin – Loading dose of 70 mg IV on day 1, then 50 mg IV once daily
- Voriconazole – Loading dose of 6 mg/kg IV every 12 hours on day 1, followed by 4 mg/kg IV every 12 hours
- Amphotericin B lipid complex – 5 mg/kg IV once daily
- Liposomal amphotericin B – 3 to 5 mg/kg IV once daily
Other azoles
- Posaconazole: Posaconazole is a broad-spectrum triazole that has been approved by the FDA for the prophylaxis of fungal infections in neutropenic patients and for the treatment of mucocutaneous candidiasis. It has in vitro activity against yeasts and molds (such as Aspergillus spp and the Mucorales), but it has not been studied for the empiric treatment of invasive fungal infections in neutropenic patients or for the treatment of established mold infections.
- Isavuconazole (isavuconazonium): Isavuconazonium is the prodrug of isavuconazole, a broad-spectrum triazole that has been approved by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis in adults. There is some experience using it for the treatment of Candida infections, but it has not been studied to date for empiric treatment in neutropenic patients.
- Itraconazole: A randomized trial compared intravenous followed by oral itraconazole to amphotericin B deoxycholate for up to 28 days as empiric therapy in 384 febrile neutropenic patients. Itraconazole was at least as effective as amphotericin B (response rate 47 versus 38 percent), and significantly fewer patients had drug-related adverse effects (5 versus 54 percent). The median duration of neutropenia in this study was 10 and 8 days for the itraconazole and amphotericin groups, respectively. Five breakthrough fungal infections were observed in each group.
- Fluconazole: Fluconazole is generally not recommended for empiric antifungal therapy because of its lack of activity against molds, a major concern in patients with hematologic malignancies and those undergoing HCT.
Colony-Stimulating Factor Therapy
Myeloid growth factors: specifically, granulocyte colony-stimulating factors (G-CSFs) and granulocyte-macrophage colony-stimulating factor (GM-CSFs)—may shorten the duration of neutropenia in patients who have undergone chemotherapy.
G-CSFs are lineage-specific for the production of functionally active neutrophils and can also be used in patients with severe, chronic neutropenia. GM-CSFs stimulate the production of neutrophils, monocytes, and eosinophils. Filgrastim and pegfilgrastim are examples of G-CSFs; sargramostim is an example of a GM-CSF. These agents are typically administered no sooner than 24 hours after chemotherapy completion. Filgrastim is often the agent of choice if a G-CSF is chosen.
The NCCN guidelines state that there is less evidence for therapeutic use than for prophylactic use. If patients with acute neutropenic fever are receiving prophylactic filgrastim or sargramostim, the CSF treatment should be continued. Pegfilgrastim should be discontinued, as it is long-acting and evidence for its therapeutic benefits is lacking. If the patient is not receiving prophylactic CSF, their risk of infection complications or poor outcome should be assessed. CSF should be considered for patients at high risk.(19)
European guidelines also recommend prophylactic treatment with G-CSFs in patients receiving chemotherapy regimens that pose a high risk of febrile neutropenia. With chemotherapy regimens associated with moderate risk, primary prophylaxis may also be advisable for cases in which patient-related factors increase the overall risk, or to maintain chemotherapy in cases where dose-dense or dose-intense chemotherapy strategies are used because of survival benefits, or reductions in chemotherapy dose intensity or density are known to be associated with a poor prognosis.(21)
Primary prophylaxis with biosimilar filgrastim has been shown to be cost-effective.(22)
Granulocyte Transfusion
Neutrophil (granulocyte) transfusions have undergone a cycle of popularity followed by disfavor. These transfusions are accompanied by many complications, including severe febrile reactions. Their use is controversial.
Although disappearing from clinical practice, granulocyte transfusions have some clinical usefulness in treating neonatal sepsis. Their usefulness in adults with neutropenia, in whom adequate increments of WBC counts are difficult to achieve, has not been demonstrated in randomized clinical trials.(23) Granulocyte transfusion could be considered in cases of gram-negative sepsis with no improvement in 24-48 hours.
GUIDELINES
To view, “Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update”, please click on below link:
http://ascopubs.org/doi/pdf/10.1200/JCO.18.00374
To view, “Outpatient Management of Fever and Neutropenia in Adults Treated for Malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical Practice Guideline Update”, please click on below link:
https://www.idsociety.org/practice-guideline/fever-and-neutropenia-in-adults-with-cancer/
To view, “IDSA Neutropenic Patients with Cancer”, please click on below link:
https://www.idsociety.org/practice-guideline/neutropenic-patients-with-cancer/
To view, “European Guidelines For Empirical Antibacterial Therapy For Febrile Neutropenic Patients In The Era Of Growing Resistance: Summary Of The 2011 4th European Conference On Infections In Leukemia”, please click on below link:
http://www.haematologica.org/content/98/12/1826.full
To view, NCCN Guidelines on Myeloid Growth Factors 2015”, please click on below link:
http://www.nccn.org/professionals/physician_gls/pdf/myeloid_growth.pdf
To view, Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update”, please click on below link:
http://jco.ascopubs.org/content/33/28/3199
CONSULTATION AND LONG TERM MONITORING
Request a hematology consultation for review of the bone-marrow slides and peripheral blood smears to confirm the diagnosis and to assist in G-CSF dosing and evaluation.
Request an infectious disease consultation for advice and assistance in the selection of appropriate antibiotics, especially in patients with complicated infections or prolonged neutropenic fever that is not responding to standard therapy.
Obtain daily CBC counts with manual differential to monitor the neutropenic patient’s recovery from an etiologic agent or to monitor the neutropenia’s response to G-CSF or GM-CSF.
If septic shock occurs, the patient should be transferred to the ICU. Intubation may be required.
PREVENTION
Effective infection prevention strategies for low-risk neutropenic patients have focused upon the most common pathogens causing the infections, including pyogenic bacteria, viruses, and fungi.
Antibacterial prophylaxis: Fluoroquinolone-based antibacterial prophylaxis has been effective in reducing febrile events and invasive gram-negative infections in high-risk neutropenic patients. Given the increased costs, drug-related adverse effects, susceptibility to superinfection (such as Clostridioides [formerly Clostridium] difficile infection), and selection for antimicrobial resistance, it is not recommended the routine use of antibacterial prophylaxis for low-risk patients for whom the duration of neutropenia (absolute neutrophil count [ANC] <500 cells/microL) is anticipated to be seven days or fewer.
Patients with solid tumors who are receiving conventional chemotherapy are generally considered to be at low risk for complications of neutropenia and therefore do not require antibacterial prophylaxis.
Antifungal prophylaxis: The risk for invasive fungal infection due to opportunistic yeasts, such as Candida spp, or molds, such as Aspergillus spp, is very low among patients for whom the anticipated duration of neutropenia (ANC <500 cells/microL) is anticipated to be seven days or fewer. Accordingly, antifungal prophylaxis for patients with solid tumors or lymphoma undergoing conventional chemotherapy with or without concomitant immunotherapies is not recommended.
Pneumocystis prophylaxis: Most low-risk cancer patients do not require prophylaxis against Pneumocystis jirovecii (formerly P. carinii) pneumonia (PCP).
Antiviral prophylaxis: Several preventive measures can be used to prevent viral infections in neutropenic patients. Hand hygiene and cough etiquette remain the most important methods for preventing the spread of respiratory virus infections in ambulatory low-risk cancer patients.
HSV and VZV: Antiviral prophylaxis against herpes simplex virus (HSV) and varicella-zoster virus (VZV) is generally not used in low-risk neutropenic patients since the rate of reactivation of these viruses is low in such patients. In contrast, antiviral prophylaxis with activity against HSV and VZV is used routinely in high-risk neutropenic patients (e.g. patients receiving induction chemotherapy for acute leukemia, hematopoietic cell transplant recipients).
Influenza: Prevention of influenza virus should be considered well in advance of the development of neutropenia. Annual immunization with a trivalent inactivated influenza vaccine is recommended for all patients being treated for cancer. Although the optimal timing for such immunization has not been established, the vaccine is generally administered >2 weeks before chemotherapy starts or, when circumstances dictate, between chemotherapy cycles and at least seven days after the last cycle. Annual immunization of all family members and other close contacts is also recommended.
Hepatitis B: Solid tumor and lymphoma patients receiving chemotherapy who have a history of previous hepatitis B virus infection are at risk of reactivation with a flare of hepatitis that may potentially result in hepatic failure. Patients with elevated circulating hepatitis B DNA or detectable levels of circulating hepatitis B surface antigen (HBsAg) are at particular risk. Those who have been infected and cleared the virus from the circulation and developed antibody to HBsAg or to hepatitis B core antigen are also at risk. Antiviral prophylaxis should be considered for such patients at risk for reactivation; when used, antiviral prophylaxis should be started one week before chemotherapy begins and continued for at least six months after the completion of chemotherapy. This strategy can reduce the risk for reactivation from 24 to 53 percent to 0 to 5 percent.
Colony stimulating factors: The Infectious Diseases Society of America guidelines recommend that the prophylactic use of colony stimulating factors (also known as myeloid growth factors or hematopoietic growth factors), such as granulocyte and granulocyte-macrophage colony stimulating factors, be considered for patients in whom the anticipated risk of fever and neutropenia is ≥20 percent.(1)
Dietary restrictions: Neutropenic patients should follow the following dietary restrictions:
- Avoid raw and undercooked meat or well water
- Commercial fruit juices, beer, milk, and milk products should be pasteurized
- Aged cheese and cheese-based dressings should not be used
- Avoid unwashed raw fruits and vegetables; these may contain large numbers of bacteria. All food should be cooked. Fresh flowers should be avoided as well
- Outdated products and all moldy products should not be consumed
- In patients with periodontitis and stomatitis, a soft or full liquid diet is indicated. Spicy and acidic foods should be avoided until recovery is complete.
Other measures: General measures to be taken include the following:
- Removal of any offending drugs or agents is the most important step in most cases involving drug exposure; if the identity of the causative agent is not known, stop administration of all drugs until the etiology is established.
- Use careful oral hygiene to prevent infections of the mucosa and teeth; control oral and gingival lesion pain with saline and hydrogen peroxide rinses and local anesthetic gels and gargles.
- Avoid rectal temperature measurements and rectal examinations.
- Administer stool softeners for constipation.
- Use good skin care for wounds and abrasions; skin infections should be managed by someone with experience in the treatment of infection in neutropenic patients.
REFERENCES
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52:427-31
- Klastersky J. Management of fever in neutropenic patients with different risks of complications. Clin Infect Dis 2004; 39 Suppl 1:S32-7
- Weycker D, Li X, Edelsberg J, Barron R, Kartashov A, Xu H, Lyman GH.. Risk and Consequences of Chemotherapy-Induced Febrile Neutropenia in Patients With Metastatic Solid Tumors. J Oncol Pract 2015; 11:47-54
- Liu CY, Lai YC, Huang LJ, Yang YW, Chen TL, Hsiao LT, Liu JH, Gau JP, Chen PM, Tzeng CH, et al. Impact of bloodstream infections on outcome and the influence of prophylactic oral antibiotic regimens in allogeneic hematopoietic SCT recipients. Bone Marrow Transplant 2011; 46:1231-9
- Penack O, Becker C, Buchheidt D, Christopeit M, Kiehl M, von Lilienfeld-Toal M, Hentrich M, Reinwald M, Salwender H, Schalk E, et al. Management of sepsis in neutropenic patients: 2014 updated guidelines from the Infectious Diseases Working Party of the German Society of Hematology and Medical Oncology (AGIHO). Ann Hematol 2014; 93:1083-95
- Jeddi R, Achour M, Amor RB, Aissaoui L, Bouteraa W, Kacem K, Lakhal RB, Abid HB, BelHadjAli Z, Turki A, et al. Factors associated with severe sepsis: prospective study of 94 neutropenic febrile episodes. Hematology 2010; 15:28-32
- Kang CI, Song JH, Chung DR, Peck KR, Ko KS, Yeom JS, Ki HK, Son JS, Lee SS, Kim YS, et al. Risk factors and pathogenic significance of severe sepsis and septic shock in 2286 patients with gram-negative bacteremia. J Infect 2011; 62:26-33
- Kar M, Biswas S. Oncological emergencies. JIACM. 2008;9(2):120-6.Kar M, Biswas S. Oncological emergencies. JIACM. 2008;9(2):120-6.
- Christie DJ. Specificity of drug-induced immune cytopenias. Transfus Med Rev 1993; 7:230.
- Tesfa D, Keisu M, Palmblad J. Idiosyncratic drug-induced agranulocytosis: possible mechanisms and management. Am J Hematol 2009; 84:428
- Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 Update by the Infectious Diseases Society of America, CID 2011:52 (15 February), page e61
- Boxer LA. How to approach neutropenia. Hematology Am Soc Hematol Educ Program 2012; 2012:174.Boxer LA. How to approach neutropenia. Hematology Am Soc Hematol Educ Program 2012; 2012:174
- National Cancer Institute. Common toxicity criteria, version 2.0. Available from URL: http://ctep.cancer.gov/forms/ CTCv20_4-30-992.pdf [access date January 3, 2003].
- Watts RG. Neutropenia. In: Wintrobe’s Clinical Hematology, 10th Ed, (Lee GR, Foerster J, Lukens J, Eds). Baltimore, Williams and Wilkins, 1999; vol 2, pp 1860–1888.
- Cnet. Neutropenia. Available from: http://www.cancer.net/navigating-cancer-care/side-effects/neutropenia (Accessed on 2019 Feb 4)
- John R Wingard. Diagnostic approach to the adult cancer patient with neutropenic fever. Uptodate. Available from: https://www.uptodate.com/contents/diagnostic-approach-to-the-adult-cancer-patient-with-neutropenic-fever?topicRef=13951&source=see_link (Accessed on 2019 Feb 4)
- John R Wingard. Treatment of neutropenic fever syndromes in adults with hematologic malignancies and hematopoietic cell transplant recipients (high-risk patients). Uptodate. Available from: https://www.uptodate.com/contents/treatment-of-neutropenic-fever-syndromes-in-adults-with-hematologic-malignancies-and-hematopoietic-cell-transplant-recipients-high-risk-patients?topicRef=16708&source=see_link (Accessed on 2019 Feb 04)
- Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2015; 33:3199.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Myeloid Growth Factors. Available at http://www.nccn.org/professionals/physician_gls/PDF/myeloid_growth.pdf. Version 2.2018 — August 03, 2018; Accessed: September 17, 2018
- Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2015 Oct 1. 33 (28):3199-212
- Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011 Jan. 47 (1):8-32
- Wang XJ, Tang T, Farid M, Quek R, Tao M, Lim ST, et al. Routine Primary Prophylaxis for Febrile Neutropenia with Biosimilar Granulocyte Colony-Stimulating Factor (Nivestim) or Pegfilgrastim Is Cost Effective in Non-Hodgkin Lymphoma Patients undergoing Curative-Intent R-CHOP Chemotherapy. PLoS One. 2016. 11 (2):e0148901
- Massey E, Paulus U, Doree C, Stanworth S. Granulocyte transfusions for preventing infections in patients with neutropenia or neutrophil dysfunction. Cochrane Database Syst Rev. 2009 Jan 21. CD005341.
