EPIDEMIOLOGY
Chemotherapy-induced nausea and vomiting (CINV) remains a significant source of distress and dissatisfaction among patients receiving cancer treatment. Prior to the introduction of 5HT3 receptor antagonists, the incidence rate of acute nausea and vomiting was approximately 40 % after cisplatin regimens and delayed nausea and vomiting was experienced by 93 % of the patients.(1) Sixty-eight percent of patients reported both nausea and vomiting; 19 % reported only nausea; 6 % reported only vomiting.(1) In the era of treatment with dexamethasone and 5HT3 antagonists, as well as NK-1 receptor antagonists, the rates of delayed nausea dropped to around 50 %, with delayed emesis in around 30–50 % of patients.(2-4)
PATHOPHYSIOLOGY
Considerable progress has been made in elucidating the mechanisms by which chemotherapy initiates the emetic reflex. This understanding includes both the anatomy and neurotransmitters involved in CINV.
Neuroanatomy: Several areas in the central and peripheral nervous systems and gastrointestinal tract are involved in CINV. Three areas in the brainstem are thought to play critical roles in the emetic reflex: the central pattern generator (CPG; previously called the “vomiting center”) and two areas in the dorsal brainstem called the nucleus tractus solitarius (NTS) and area postrema (AP).(5-8)

- The CPG is an anatomically indistinct collection of receptor and effector nuclei located in the medulla that coordinates the emetic reflex. It appears to coordinate the efferent respiratory, gastrointestinal, and autonomic activity associated with nausea and vomiting and functions as the final effector pathway through which a variety of afferent stimuli can activate emesis.(9-10)
- The AP and NTS form a circumventricular structure located at the caudal end of the fourth ventricle. Together, these two areas were previously referred to as the “chemoreceptor trigger zone.” This part of the dorsal brain stem lies outside of the blood-brain barrier and is, therefore, accessible to emetic stimuli borne either in the blood or cerebrospinal fluid.(6) The AP/NTS appears to be an important source of afferent input to the CPG and is an important site for muscarinic (M1), dopamine (D2), serotonin (5-hydroxytryptamine [5-HT]), neurokinin-1 (NK1), and histamine (H1) receptors.(11-12)
Two other sources of afferent input to the CPG with CINV include higher brainstem and cortical structures, which may play a role in anticipatory emesis, and input from the gastrointestinal tract that is conveyed by the vagus and splanchnic nerves, whose afferents terminate in the NTS in close proximity to or within the AP itself.(13,14) These two areas of the brainstem are referred to collectively as the dorsal vagal complex.
Neurotransmitters: Although over 30 neurotransmitters have been associated with the peripheral and central nervous system sites involved in CINV, three neurotransmitters have the most clinical relevance, since therapeutic agents designed to antagonize their action have shown clinical benefit as antiemetics:(15)
- D2
- 5-HT
- Substance P
5-HT received significant attention during the 1980s in preclinical studies attempting to elucidate the mechanisms of CINV.(16) These studies led to the development of the type-three 5-HT (5-HT3) receptor antagonists during the early 1990s,(17) and these drugs have become a mainstay of current antiemetic therapy for CINV.
Substance P became a focus for preclinical studies evaluating the emetic reflex beginning in the 1990s. Substance P binds to the NK1 receptors,(18) and selective antagonists of the NK1 receptor are potent antiemetics in preclinical models using a variety of emetic stimuli.(19) Subsequent clinical trials in CINV led to the development of aprepitant and fosaprepitant for patients receiving highly emetogenic chemotherapy.
Mechanisms: A precise understanding of the mechanisms by which chemotherapy induces emesis remains elusive. Several reviews summarize our current understanding of the pathophysiology of CINV.(20-25) The following figure schematically illustrates potential sites at which chemotherapy is thought to exert its emetic effects.
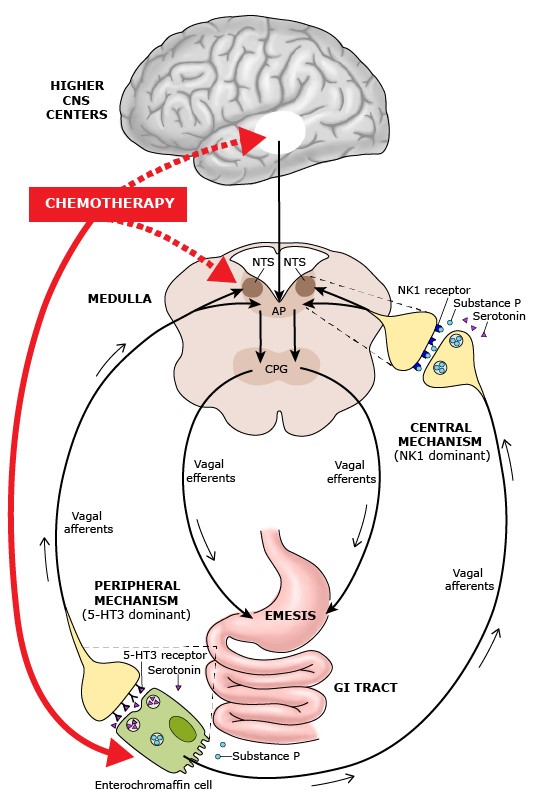
Pathways by which chemotherapeutic agents may produce an emetic response(26) Courtesy Uptodate
The mechanism that is best supported by research is that chemotherapeutic drugs can cause nausea and vomiting by activating neurotransmitter receptors in the small intestine; afferent fibers then transmit the stimuli to the brainstem, which then sends out efferent signals via the vagus nerve to induce vomiting.
Current knowledge suggests that the emetic response to chemotherapy can occur through a central and a peripheral pathway, mediated by different neurotransmitter:(27,28)
- The peripheral pathway, which is predominantly mediated by 5-HT and 5-HT3 receptors in the intestinal tract, is associated primarily with acute emesis (that occurring within 24 hours of chemotherapy).
- A central pathway, which is predominantly mediated by Substance P and NK1 receptors and is located primarily in the brain, is activated later and mainly associated with delayed CINV (after 24 hours).
Some chemotherapy agents or their metabolites may interact directly or indirectly with receptors within the AP/NTS, with subsequent activation of the CPG. Furthermore, higher central nervous system centers located in structures in the limbic forebrain, such as the amygdala, may also be a source of some types of emetic stimuli.
SIGN AND SYMPTOMS(29)
Nausea can be described as an unpleasant or queasy feeling causing a desire to vomit. It is important to note that nausea is not always accompanied by vomiting. The act of vomiting can be defined as the expulsion of gastric content through the mouth.
Symptoms that may accompany nausea include
- Tachycardia
- Perspiration
- Light-Headedness
- Dizziness
- Pallor
- Excess Salivation
- Anorexia
- Weakness
DIFFERENTIAL DIAGNOSIS(29)
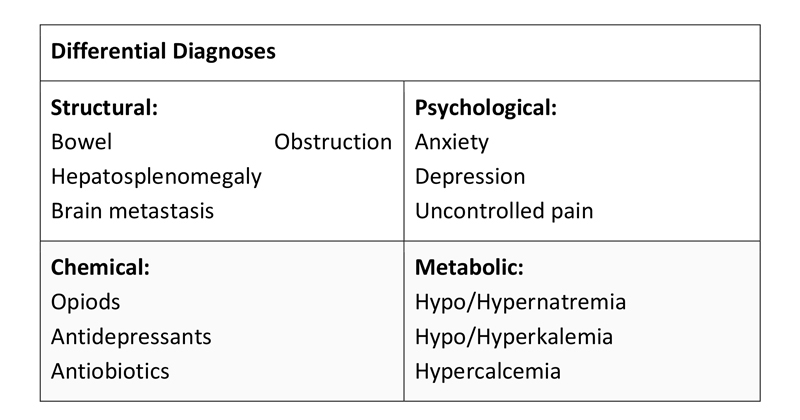
Nausea and vomiting in cancer patients can be multi-factorial. Evaluation of symptoms should reflect this. Nausea and vomiting can have structural, psychological, chemical, metabolic or a combination of origins. When evaluating cancer patients with suspected CINV, causes such as pain, anxiety, hepatosplenomegaly, bowel obstruction, metastasis or increased ICP should also be considered. Immunocompromised and elderly patients should also be evaluated for bacterial or viral gastroenteritis as these populations may be more vulnerable to an infectious process. It is crucial to illicit onset and duration as well as any potential associated, aggravating or relieving symptoms.
TREATMENT OPTIONS
Treatment recommendations are based on four types of chemotherapy-induced nausea and vomiting (CINV), as follows:
- Acute: Onset of emesis within a few minutes to several hours after chemotherapy is administered and usually peaking in the first 4-6 hours
- Delayed: Onset of emesis more than 24 hours after chemotherapy is administered
- Anticipatory: Onset of emesis prior to chemotherapy administration as a conditioned response in patients who have experienced emesis during a previous cycle of chemotherapy
- Breakthrough/refractory: Emesis despite prophylactic/breakthrough medications
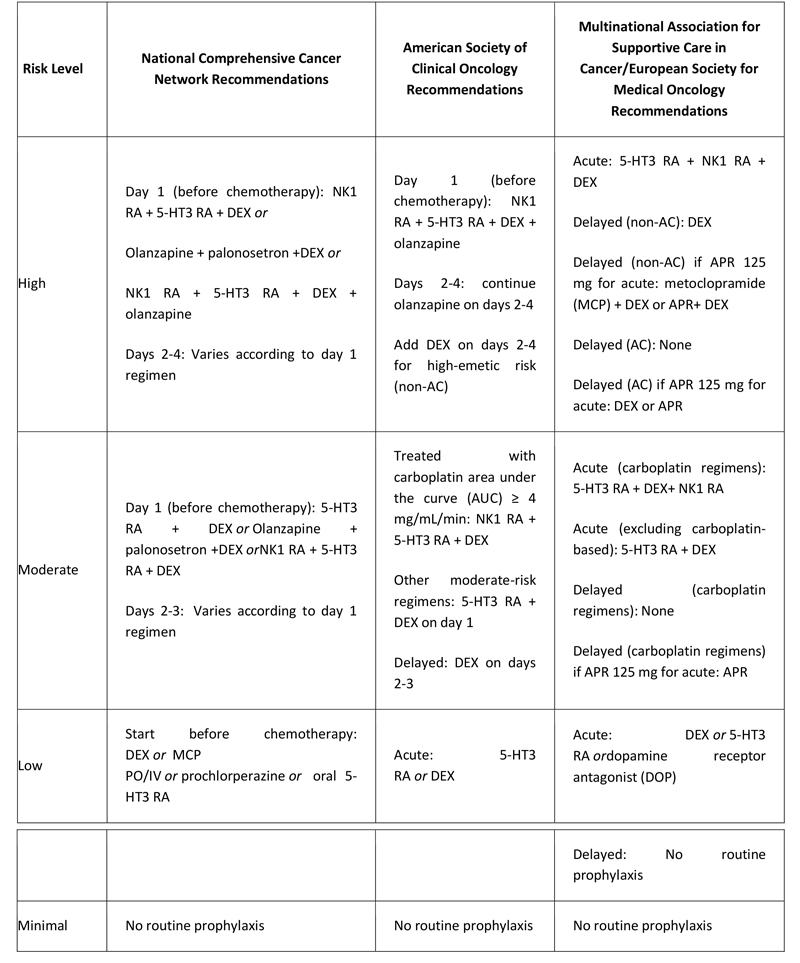
All the guidelines use the following system to classify chemotherapy agents into the following risk groups:
- High emetic risk: ≥90% or more of patients experience emesis
- Moderate emetic risk: 30% to 90% of patients experience emesis
- Low emetic risk: 10% to 30% of patients experience emesis
- Minimal emetic risk: < 10% of patients experience emesis
The Multinational Association of Supportive Care in Cancer (MASCC) / European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology (ASCO) risk ratings for individual chemotherapy agents are similar,(30) as are the NCCN risk ratings, although the National Comprehensive Cancer Network (NCCN) list of agents is more extensive.(31) Single-agent cisplatin and the combination of an anthracycline and cyclophosphamide (AC) are rated at high risk by all three organizations.(30-32) ASCO and NCCN recommend that in patients receiving combination chemotherapy, antiemetic treatment should be determined according to the agent posing the greatest degree of emetic risk.(31,32)
NCCN guidelines state that the best management of acute or delayed CINV is prevention; patients should be protected before receiving chemotherapy and for the full period of risk (up to 4 days) afterward. General recommendations for prevention are as follows:(31)
- Prevention of acute emesis should start before chemotherapy and continue for the first 24 hours
- Choice of antiemetic agent should be based on the emetic risk of the chemotherapy regimen
- Prevention of delayed emesis is a continuation of prophylactic treatment for 2 to 4 days following completion of chemotherapy
- Because breakthrough/refractory emesis is difficult to reverse, prevention using routine around-the-clock administration of antiemetics is preferred over as-needed (PRN) dosing
- Prevention is also key to the management of anticipatory emesis
- Relaxation/systematic desensitization, hypnosis with guided imagery, and music therapy are behavioral interventions that may be considered for anticipatory emesis; acupuncture/acupressure are additional options
- Consider using lorazepam as an adjuvant to the antiemetic regimen to decrease anxiety in patients at risk for anticipatory emesis
- Consider using an H2 blocker or a proton pump inhibitor to prevent dyspepsia
- Consider other potential causes of emesis in cancer patients (e.g. bowel obstruction)
Antiemetic agents
Neurokinin 1 receptor antagonist (NK1 RA):
- Aprepitant (APR) PO/IV
- Fosaprepitant (FOS)
- Netupitant/palonosetron (fixed NK1 RA and selective 5-hydroxytryptamine receptor antagonist [5-HT3 RA] combination)
5-HT3 RA:
- Dolasetron
- Granisetron PO/IV/transdermal
- Ondansetron
- Palonosetron
- Dexamethasone (DEX) PO/IV
- Olanzapine PO – Off-label use for prevention of chemotherapy associated nausea or vomiting in combination with 5-HT3 antagonist and DEX(33)
Recommended antiemetic agents by emetic-risk categories are shown in the table below.
Table. Antiemetic Treatment Recommendations
Breakthrough treatment
NCCN guidelines advise that the general principle of breakthrough treatment is to add one agent from a different class than in the patient’s current regimen. Any of the following may be selected:(31)
- Olanzapine
- Lorazepam
- Cannabinoid (eg, dronabinol, nabilone)
- Haloperidol
- Metoclopramide
- Scopolamine transdermal
- Phenothiazine (eg, promethazine, prochlorperazine)
- 5-HT3 RA
- DEX
Oncology Nursing Society Recommendations
The ONS practice recommendations for CINV in adults include the following:(34)
- Cannabis/cannabinoids
- Dexamethasone-sparing regimen
- Netupitant-palonosetron combination (NEPA)
- NK1 RA
- Olanzapine
- 5-HT3 RA
- Sustained-release granisetron
- Transdermal granisetron
- Triple drug regimen: NK1 RA + 5-HT3 RA + DEX
In addition, the following treatments were considered by ONS likely to be effective:
- Adherence to CINV guidelines
- Benzodiazepine for anticipatory CINV
- Gabapentin
- Hypnosis for anticipatory CINV
- Managing patient expectations
- Olanzapine for breakthrough CINV
- Oral palonosetron
- Progestins
- Progressive muscle relaxation and guided imagery
- Single-agent dexamethasone
The ONS considered the benefits balanced with harm for virtual reality, which has been evaluated for providing distraction and reducing anxiety as means of CINV prevention.
The effectiveness of the following treatments was not considered established:
- Acupressure
- Acupuncture/electroacupuncture
- Acustimulation
- Aromatherapy
- Lorazepam [Ativan], diphenhydramine [Benadryl], and haloperidol [Haldol] gel (ABH gel)
- Ayurvedic drugs
- Carbamazepine
- Chamomilla recutita
- Electronic antinausea device (ELANI)
- Exercise
- Ginger
- Grape Juice
- Guided imagery/imagery alone
- Haloperidol
- Herbal medicine
- Hologram bracelet
- Institutional initiatives
- Massage/aromatherapy massage
- Metoclopramide (prophylactic)
- Metopimazine
- Mirtazapine
- Nevasic audio
- Ondansetron as rescue medication
- Prochlorperazine for breakthrough CINV
- Progressive muscle relaxation (PMR)
- Psychoeducation/psychoeducational interventions
- Thalidomide
- Therapeutic touch
- Yoga
The ONS also found cocculine unlikely to be effective and that expert opinion supports the adjunctive use of lorazepam for CINV prevention.
GOALS OF THERAPY
Goal of therapy is to control and manage the CINV during acute and delayed phase to minimize the discomfort to patient on chemotherapy.
GUIDELINES
To review, “NCCN guidelines: Insights Antiemesis, Version 2.2017”, please click on below link:
http://www.jnccn.org/content/15/7/883.full.pdf+html
To review, “American Society of Clinical Oncology (ASCO) : New Recommendations for Controlling Nausea and Vomiting Related to Cancer Treatment 2017”, please click on below link:
https://www.asco.org/about-asco/press-center/news-releases/new-recommendations-controlling-nausea-and-vomiting-related
To review, “2016 Multinational Association for Supportive Care in Cancer (MASCC) / European Society for Medical Oncology (ESMO) guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients”, please click on below link:
https://www.esmo.org/Guidelines/Supportive-and-Palliative-Care/MASCC-and-ESMO-Consensus-Guidelines-for-the-Prevention-of-Chemotherapy-and-Radiotherapy-Induced-Nausea-and-Vomiting
To view, “Oncology Nursing Society (ONS) guidelines on Chemotherapy-Induced Nausea and Vomiting—Adult”, please click on below link:
https://www.ons.org/pep/chemotherapy-induced-nausea-and-vomiting-adult
PRECAUTIONS(35)
Advice following preventions to the patients:
Fluids:
- Drink fluids throughout the day like water and juices. Many persons on chemotherapy need to drink at least two quarts of fluids per day. Also, if you are vomiting it is important to replace the fluids lost to avoid getting dehydrated.
- Avoid drinking liquids at meals.
Eating hints:
- Eat small amounts of food throughout the day.
- Eat before you get too hungry.
- Eat dry foods such as dry cereal, toast, or crackers without liquids especially first thing in the morning.
- Avoid heavy, high fat and greasy meals right before chemotherapy.
- Do not eat your favorite foods during this time. They will no longer be favorite foods if you begin to associate them with nausea and vomiting episodes.
Surroundings:
- Avoid strong odors.
- Don’t lay flat for at least two hours after eating. Rest by sitting up or reclining with your head elevated.
- Fresh air and loose clothing may be helpful after eating.
- Exercising after eating may slow down digestion and increase discomfort.
Distraction:
- Relax and try to keep your mind off the chemotherapy.
Other ways to minimize chemotherapy nausea:
- If you are vomiting, stop eating. Once you stop vomiting, start back on food slowly. Start with small amounts of clear liquids, such as broth, juice soda, sports drinks, or water. Then, advance to light, mild foods like jello, bananas, rice, or toast. Soon, you will be back to solid foods.
- Avoid caffeine and smoking.
- Suck on hard candy, popsicles, or ice during chemotherapy.
REFERENCES
- Kris MG, Gralla RJ, Clark RA, Tyson LB, O’Connell JP, Wertheim MS, Kelsen DP. Incidence, course, and severity of delayed nausea and vomiting following the administration of high-dose cisplatin. J Clin Oncol Off J Am Soc Clin Oncol. 1985;3(10):1379–1384.
- Campos D, Pereira JR, Reinhardt RR, Carracedo C, Poli S, Vogel C, Martinez-Cedillo J, Erazo A, Wittreich J, Eriksson LO, Carides AD, Gertz BJ. Prevention of cisplatin-induced emesis by the oral neurokinin-1 antagonist, MK-869, in combination with granisetron and dexamethasone or with dexamethasone alone. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19(6):1759–1767.
- Hesketh PJ, Gralla RJ, Webb RT, Ueno W, DelPrete S, Bachinsky ME, Dirlam NL, Stack CB, Silberman SL. Randomized phase II study of the neurokinin 1 receptor antagonist CJ-11, 974 in the control of cisplatin-induced emesis. J Clin Oncol Off J Am Soc Clin Oncol. 1999;17(1):338–343.
- Navari RM, Reinhardt RR, Gralla RJ, Kris MG, Hesketh PJ, Khojasteh A, Kindler H, Grote TH, Pendergrass K, Grunberg SM, Carides AD, Gertz BJ. Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist. L-754, 030 Antiemetic Trials Group. N Engl J Med. 1999;340(3):190–195. doi: 10.1056/nejm199901213400304
- Borison HL. Area postrema: chemoreceptor circumventricular organ of the medulla oblongata. Prog Neurobiol 1989; 32:351.
- Miller AD, Leslie RA. The area postrema and vomiting. Front Neuroendocrinol 1994; 15:301.
- Fukuda H, Koga T. Non-respiratory neurons in the Bötzinger complex exhibiting appropriate firing patterns to generate the emetic act in dogs. Neurosci Res 1992; 14:180.
- Koga T, Fukuda H. Neurons in the nucleus of the solitary tract mediating inputs from emetic vagal afferents and the area postrema to the pattern generator for the emetic act in dogs. Neurosci Res 1992; 14:166.
- Carpenter DO. Neural mechanisms of emesis. Can J Physiol Pharmacol 1990; 68:230.
- Miller AD, Wilson VJ. ‘Vomiting center’ reanalyzed: an electrical stimulation study. Brain Res 1983; 270:154.
- Mitchelson F. Pharmacological agents affecting emesis. A review (Part I). Drugs 1992; 43:295.
- Bountra C, Gale JD, Gardner CJ, et al. Towards understanding the aetiology and pathophysiology of the emetic reflex: novel approaches to antiemetic drugs. Oncology 1996; 53 Suppl 1:102.
- BORISON HL, WANG SC. Physiology and pharmacology of vomiting. Pharmacol Rev 1953; 5:193.
- Wang SC. Emetic and antiemetic drugs. In: Physiological pharmacology: A comprehensive treatise, Root WS, Hofmann FG (Eds), Academic Press, New York 1965. Vol Vol II, p.225.
- Leslie RA. Neuroactive substances in the dorsal vagal complex of the medulla oblongata: nucleus of the tractus solitarius, area postrema, and dorsal motor nucleus of the vagus. Neurochem Int 1985; 7:191
- Andrews PL, Rapeport WG, Sanger GJ. Neuropharmacology of emesis induced by anti-cancer therapy. Trends Pharmacol Sci 1988; 9:334
- Hesketh PJ, Gandara DR. Serotonin antagonists: a new class of antiemetic agents. J Natl Cancer Inst 1991; 83:613
- Saria A. The tachykinin NK1 receptor in the brain: pharmacology and putative functions. Eur J Pharmacol 1999; 375:51
- Watson JW, Gonsalves SF, Fossa AA, et al. The anti-emetic effects of CP-99,994 in the ferret and the dog: role of the NK1 receptor. Br J Pharmacol 1995; 115:84
- Hesketh PJ. Understanding the pathobiology of chemotherapy-induced nausea and vomiting. Providing a basis for therapeutic progress. Oncology (Williston Park) 2004; 18:9.
- Rudd JA, Andrews PLR. Mechanisms of acute, delayed, and anticipatory emesis induced by anticancer therapies. In: Management of nausea and vomiting in cancer and cancer treatment, Hesketh PJ (Ed), Jones and Bartlett Publishers, Sudbury 2005. p.15.
- Rojas C, Slusher BS. Pharmacological mechanisms of 5-HT₃ and tachykinin NK₁ receptor antagonism to prevent chemotherapy-induced nausea and vomiting. Eur J Pharmacol 2012; 684:1.
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med 2008; 358:2482.
- Janelsins MC, Tejani MA, Kamen C, et al. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother 2013; 14:757.
- Navari RM, Aapro M. Antiemetic Prophylaxis for Chemotherapy-Induced Nausea and Vomiting. N Engl J Med 2016; 374:1356.
- Modified from Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med 2008; 358:2482.
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med 2008; 358:2482.
- Janelsins MC, Tejani MA, Kamen C, et al. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother 2013; 14:757.
- Camp-Sorrell D, Hawkins RA, editors. Clinical manual for the oncology advanced practice nurse. Oncology Nursing Society; 2014.
- Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016 Sep. 27 (suppl 5):v119-v133
- NCCN Clinical Practice Guidelines in Oncology. Antiemesis. National Comprehensive Cancer Network. Available at https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Version 2.2018 — April 30, 2018; Accessed: May 4, 2018
- Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017 Jul 31. 34 (4):JCO2017744789.
- Navari RM, Qin R, Ruddy KJ, Liu H, Powell SF, Bajaj M, et al. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting. N Engl J Med. 2016 Jul 14. 375 (2):134-42.
- Oncology Nursing Society. Chemotherapy-Induced Nausea and Vomiting—Adult. ONS. Available at https://www.ons.org/practice-resources/pep/chemotherapy-induced-nausea-and-vomiting/chemotherapy-induced-nausea-and. April 14, 2017; Accessed: May 4, 2018.
Chemocare.com. Nausea, Vomiting & Chemotherapy. http://chemocare.com/chemotherapy/side-effects/nausea vomiting-chemotherapy.aspx (accessed 17 January 2019)
